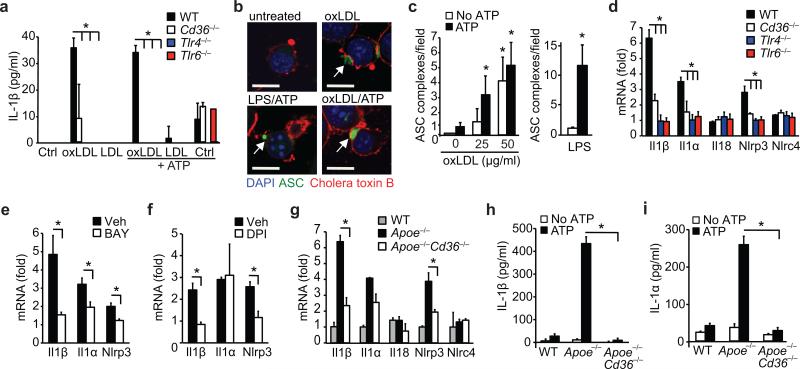
Figure 3. CD36 regulates priming of the NLRP3 inflammasome by oxLDL through TLR4-TLR6.
(a) IL-1β ELISA of supernatants from wild-type (WT), CD36-deficient (Cd36–/–), TLR4-deficient (Tlr4–/–) or TLR6-deficient (Tlr6–/–) peritoneal macrophages primed with oxLDL, unmodified LDL (50 μg/mL, 18 h) or PBS vehicle (Ctrl) and subsequently treated with ATP (5 μM, 1 h). (b) Confocal microscopy of ASC-CFP expressing immortalized macrophages treated as indicated. (c) Quantification of ASC-oligomerization (speck formation) per 20x field for the indicated treatments. (d-f) Relative mRNA expression of the indicated gene in oxLDL-treated WT, Cd36–/–, Tlr4–/– or Tlr6–/– peritoneal macrophages (d) or from WT peritoneal macrophages following pre-treatment with BAY 11-7083 (BAY, an NF-κB inhibitor, 10 μM,) (e) or diphenylene iodonium (DPI, an NAPDH oxidase inhibitor, 1 μM) (f), measured by qRT-PCR. (g) Relative mRNA expression in in-vivo formed foam cell macrophages from mice of the indicated genotype fed a western diet and expressed relative to macrophages from chow-fed mice. (h-i) IL-1β (h) or IL-1α (i) production from in-vivo formed foam cells from mice of the indicated genotype treated ex-vivo with ATP (5 μM, 1 h). Data in a, h-i are mean ± s.d. of triplicate samples within a single experiment and are representative of three independent experiments. Data in c-f and g is mean ± s.e.m. of three independent experiments. Images in b are representative of three independent experiments. Scale bar =10 μm. *P<0.05.