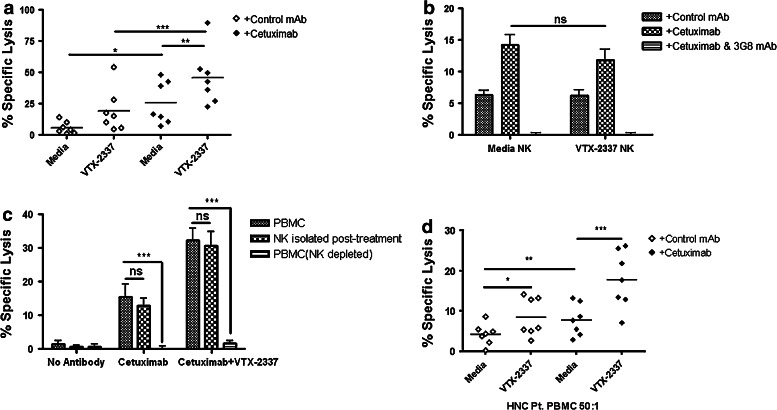
Fig. 1.
TLR8 stimulation indirectly activates NK cells to enhance cetuximab-mediated ADCC. a PBMC were incubated with TLR8 selective agonist VTX-2337 (250 nM) or with media for 18 h, then co-cultured at E:T = 50:1 for 4 h with 51Cr-labeled UM-22B HNC cells coated with control IgG1 or cetuximab. Under conditions of TLR8 stimulation of PBMC followed by incubation with cetuximab-coated HNC cells, enhanced ADCC was observed compared to unstimulated PBMC against cetuximab-coated HNC cells (p < 0.01). b Purified NK cells were treated with VTX-2337 (250 nM) or media for 18 h, then co-cultured with 51Cr-labeled UM-22B HNC cells for 4 h at an E:T = 10:1. VTX-2337 treatment did not significantly influence NK cell cytotoxicity using control IgG1 or cetuximab-coated HNC cells (p = 0.13). Blockage of FcγRIIIa by 3G8 mAb abolished ADCC activity of NK cells. c PBMC were stimulated with VTX-2337 (250 nM) or media for 18 h, and then a portion of PBMC were fractionated into purified NK cells or NK-depleted PBMC and then assayed separately for ADCC activity. PBMC (E:T = 50:1) and NK cells (E:T = 2.5:1) demonstrated similar levels of specific lysis, while NK depletion abolished cytotoxicity of PBMC. d TLR8 stimulation enhanced cetuximab-mediated ADCC in PBMC from treatment-naïve HNC patients with active disease. TLR8 stimulation treatment alone produced enhancement of ADCC in PBMC from HNC patients (p < 0.05), whereas TLR8 stimulation plus cetuximab induced significantly greater ADCC activity of PBMC (p < 0.001). Cetuximab and control IgG1 antibodies were used at 10 μg/mL. *p < 0.05, **p < 0.005, ***p < 0.001