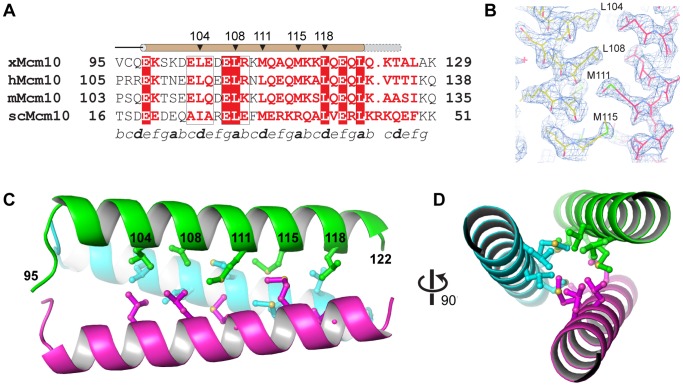
Figure 3. Crystal structure of the Mcm10 coiled-coil.
(A) Sequence alignment of the coiled-coil region from Xenopus laevis (x), Homo sapiens (h), Mus musculus (m), and Saccharomyces cerevisiae (sc) Mcm10. Red boxes indicate identical amino acids. The predicted heptad repeat pattern is labeled by letters a-g at the bottom. The position of the helix is shown schematically at the top (brown, MBP-CC95–124; grey, MBP-CC95–132). (B) Composite 2Fo−Fc omit electron density map (contoured at 1σ) with carbon atoms colored according to protomer. (C) Crystal structure of xMcm10-CC95–124. Residues at the interface are shown in ball and stick, and the amino acid numbers labeled in black. (D) View down the helical axis, rotated 90° from the view in B.