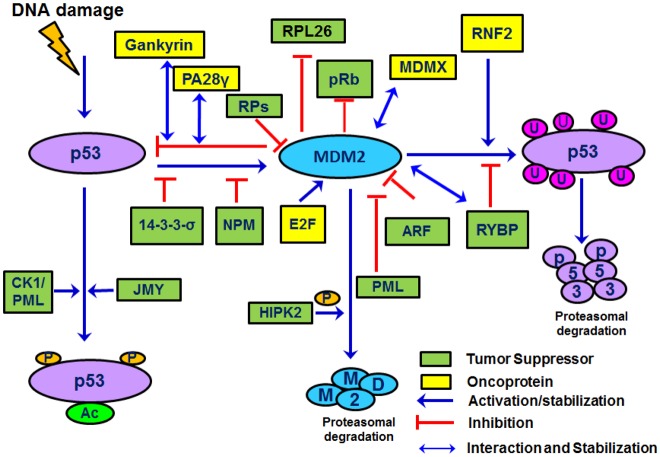
Fig. 5. Several tumor suppressors and oncoproteins regulate the MDM2-p53 interaction.
Ribosomal proteins (RP-both the large subunit and small subunits) form a complex with p53 and MDM2 to inhibit MDM2-mediated p53 ubiquitination and stabilization of p53. ARF and PML sequester the MDM2 in the nucleolus, inhibiting MDM2 from binding and degrading p53. CK1 phosphorylates p53 at Thr18 in response to stress and DNA damage and, along with p53, localizes to the PML nuclear bodies. MDMX forms heteroligomers with MDM2 and induces p53 degradation. PA28γ protein interacts with both MDM2 and p53 proteins and promotes the MDM2-p53 interaction, leading to enhanced MDM2-mediated p53 ubiquitination and degradation. RYBP interacts with MDM2 to decrease MDM2-mediated p53 ubiquitination while RNF2 promotes p53 degradation. HIPK2, tumor suppressor (Ts) protein phosphorylates MDM2, promoting its proteasomal degradation while the Rb Ts forms a ternary complex with p53 and MDM2.