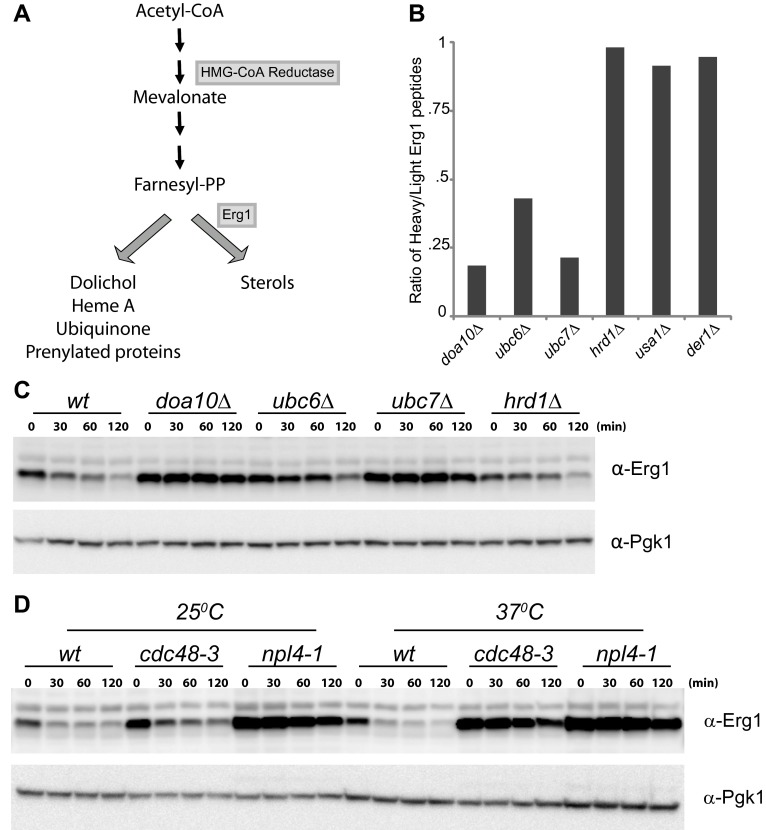
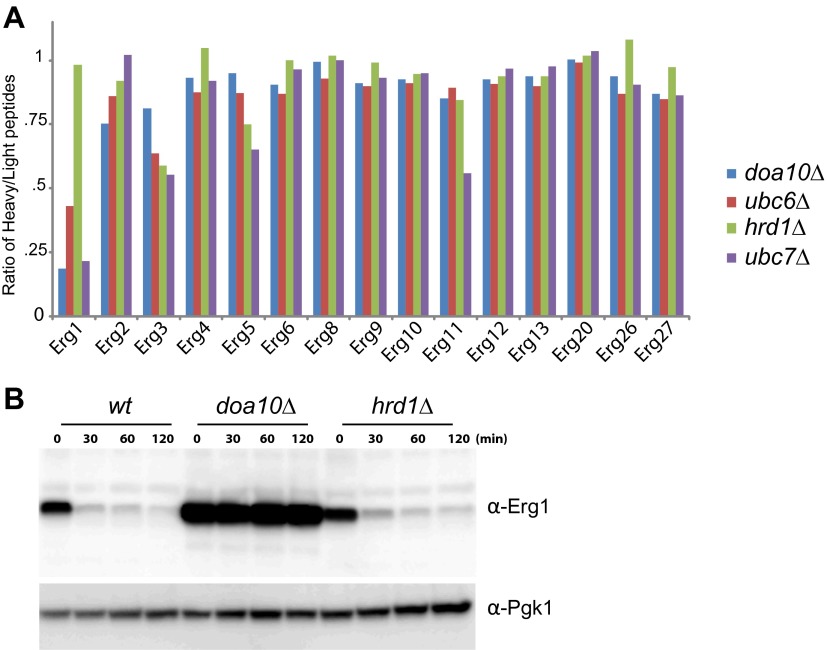
Figure 1. Erg1 is a substrate of the Doa10 complex.
(A) Schematic representation of the mevalonate pathway and its different end products. The steps catalyzed by HMG-CoA reductase and the squalene monooxygenase Erg1 are indicated. Adapted from Goldstein and Brown (1990). (B) Erg1 abundance in the indicated mutants relative to wt cells, as detected by mass spectrometry upon SILAC labeling. All strains used are lysine auxotrophs and were grown in the presence of either heavy L-lysine (wt cells) or light L-lysine (deletion mutants). Note that high steady state levels of Erg1 result in a low heavy/light ratio. (C) The degradation of endogenous Erg1 was followed after inhibition of protein synthesis by cycloheximide in wt cells or in cells with the indicated deletions. Whole-cell extracts were analyzed by SDS–PAGE and western blotting. Erg1 was detected with α-Erg1 antibody. Phosphoglycerate kinase (Pgk1) was used as loading control and detected with α-Pgk1 antibodies. A representative gel of three independent experiments is shown. (D) The degradation of endogenous Erg1 was analyzed as in (C) in wt cells or the temperature sensitive cdc48-3 and npl4-1 cells either at the permissive temperature of 25°C or after a 2 hr shift to 37°C, the restrictive temperature.