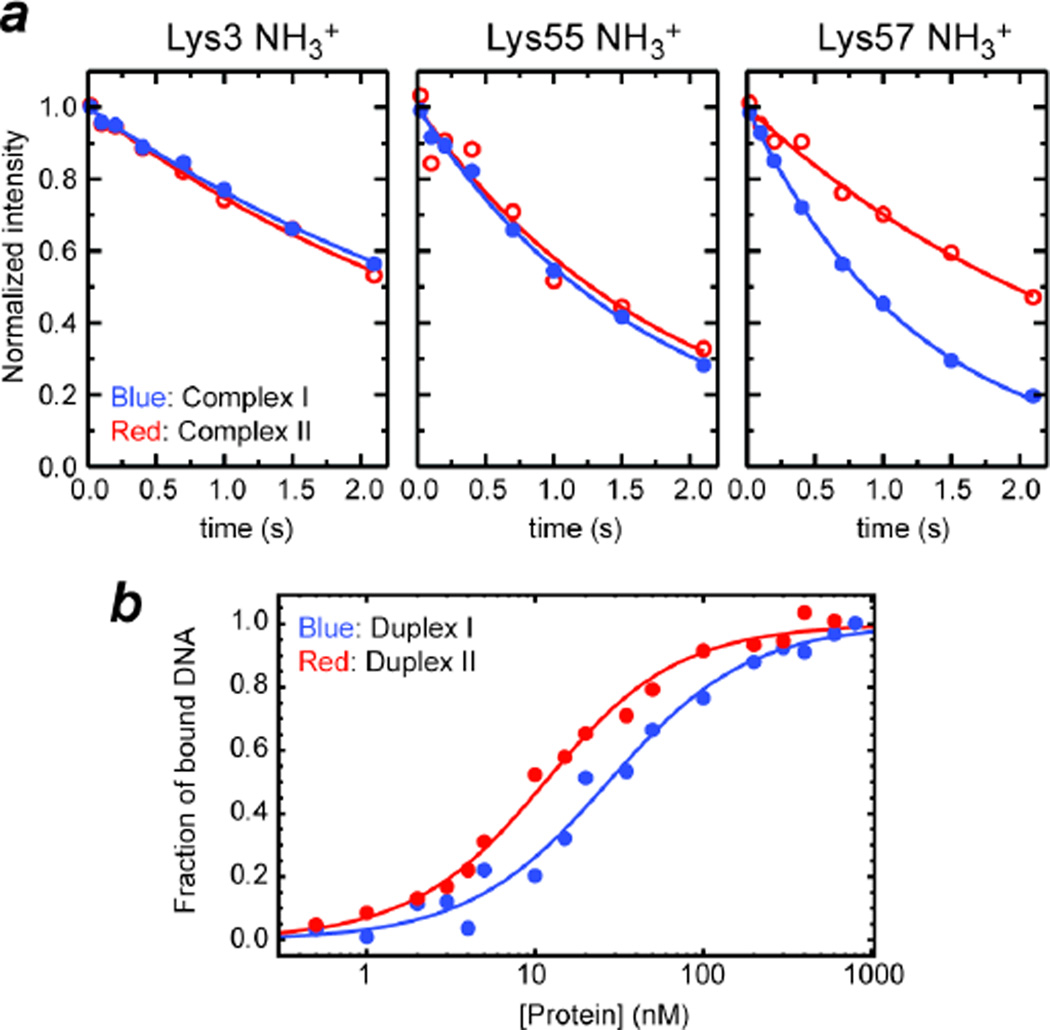
Figure 4.
Influence of the oxygen-to-sulfur substitution in the DNA phosphate group interacting with Lys57. (a) 15N longitudinal relaxation of Lys side-chain NH3+ groups at the protein-DNA interfaces of Complexes I and II. Lys57 NH3+ group exhibited substantially different relaxation upon the oxygen-to-sulfur substitution in the DNA phosphate group. 15N relaxation of the other NH3+ groups (interacting with normal phosphate group in both complexes) was virtually unaffected. (b) Binding isotherm as monitored with fluorescence arising from a rhodamine attached to the 5’-terminus of DNA. Fractions of bound DNA calculated from the fluorescence anisotropy data at varying concentrations of HoxD9 homeodomain are plotted for Duplexes I and II.