Abstract
Cholangiocytes are involved in a variety of processes essential for liver pathophysiology. To meet their demanding metabolic and functional needs, bile ducts are nourished by an own arterial supply, the peribiliary plexus. This capillary network originates from the hepatic artery and is strictly arranged around the intrahepatic bile ducts. Biliary and vascular structures are linked by a close anatomic and functional association necessary for liver development, normal organ physiology and liver repair. This strong association is finely regulated by a range of angiogenic signals, enabling the crosstalk between cholangiocytes and the different vascular cell types. This review will briefly illustrate the “vascular” properties of cholangiocytes, their underlying molecular mechanisms and the relevant pathophysiological settings.
Keywords: Cholangiocytes, peribiliary plexus, cholangiopathies, VEGF, PDGF
1. Introduction
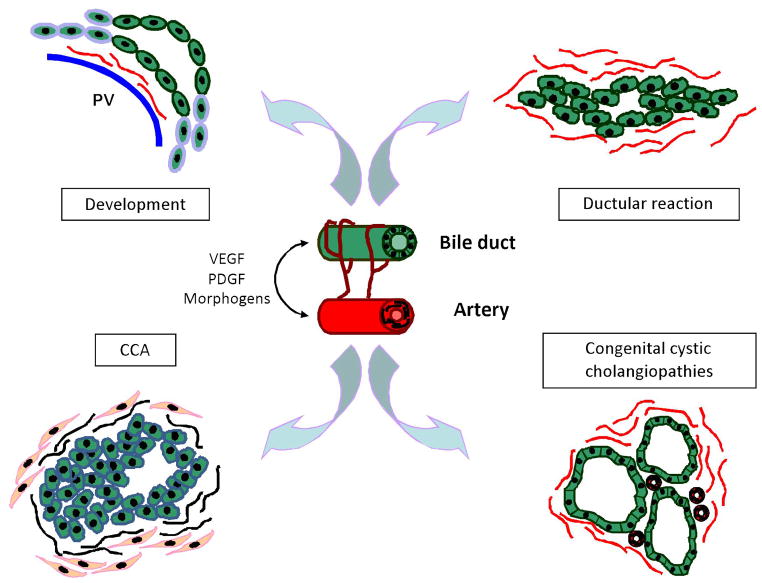
The intrahepatic biliary tree is a network of interconnecting ducts beginning with the canal of Hering, located at the portal-parenchyma interface, which progressively merges into a system of interlobular, septal and major ducts embedded into the portal space that coalesces into the extrahepatic bile ducts, finally delivering bile to the gallbladder and to the intestine. In the liver microarchitecture, bile ducts run in parallel with a branch of the portal vein and with one or two branches of the hepatic artery. This close anatomical association between the biliary and either liver vascular systems gives rise to the portal triad.1 In addition to regulating bile production through secretory and absorptive mechanisms, cholangiocytes are involved in a variety of processes essential for liver pathophysiology, including angiogenesis.2 To support the metabolic and functional needs of cholangiocytes, the biliary tree is nourished by its own arterial supply, forming the peribiliary plexus (PBP). The PBP originates from the hepatic artery and is strictly arranged around the intrahepatic bile ducts.3 Bile ducts establish an intimate anatomic and functional association with the arterial vasculature in liver development as well as in liver repair, as summarized in Figure 1. This association is finely regulated by signals exchanged between cholangiocytes and vascular cell types, including endothelial and mural cells.
Figure 1. Association between biliary and vascular cells in the liver.
The intimate anatomic and functional association between bile ducts and PBP is finely regulated by a variety of signaling pathways – including angiogenic factors. Changes in the biliary tree architecture are closely linked to vascular proliferation in various pathophysiological settings. During development, hepatic artery branches are formed in close proximity to the nascent ductal plates; following a cholestatic damage, ductular reaction is characterized by cholangiocytes proliferation, along with a massive adaptive vascular response; angiogenic factors play a major role in neoplastic cholangiopathies such as CCA for the generation of the tumor reactive stroma; congenital cystic cholangiopathies are characterized by a rich vascularization, necessary to support abnormal growth of the cystic epithelium.
2. Liver development
The liver develops from a diverticulum of the ventral endoderm. The endodermal cells located in the cranial portion of the liver bud specify into hepatoblasts, giving rise to both types of liver epithelial cells, i.e. the hepatocyte and the cholangiocyte. The development of the biliary epithelium follows the portal vein system: the hepatoblasts located in contact with the mesenchyme containing the peripheral branches of the portal vein (portal mesenchyme), switch their phenotype to form a single layered ring of small flat epithelial cells, called ductal plate, the primordial structure from which the bile ducts originate. Focal areas of the ductal plate are then duplicated by a second layer of cells. These double-layered structures show an asymmetrical organization, lined by cells with a cholangiocyte phenotype on the portal side and by cells with a hepatoblast phenotype on the parenchymal side.4 Once a lumen has been formed, the nascent bile duct is entirely lined by cholangiocytes; this tubular structure further dilates to form the immature bile duct progressively migrating into the portal mesenchyme. As the portal space develops, biliary tubules are remodelled into individual bile ducts, fully incorporated into the portal mesenchyme, which proliferate and elongate to expand the biliary tree until maturation is completed.4–6 Bile duct morphogenesis is closely coupled with the hepatic artery development, since hepatic artery branches are formed in close proximity to the nascent ductal plates.7
The relevance of these duct-artery relationships is highlighted by ductal plate malformations (DPM). These congenital cholangiopathies are characterized by developmental anomalies, in which dysmorphic bile ducts are strictly associated with an abnormal ramification of vascular structures, resembling a “polard willow” configuration.8, 9 Similar anomalies in bile ducts and artery development can be observed in mice lacking Hnf6 or Hnf1β genes, encoding for transcription factors critically involved in biliary development. The inactivation of these genes leads to artery dysgenesis, in strict conjunction with bile duct abnormalities.5, 10, 11 As detailed below, biliary morphogenesis is driven by a variety of signaling pathways, including angiogenic factors, which are normally switched off when maturation is completed, but that can be reactivated in a variety of disease conditions.
3. Liver repair
Cholangiocytes are able to proliferate in response to biliary damage. The expansion of the epithelial compartment in the liver is a key step in the repair mechanisms aimed at compensating the anatomical and functional loss of injured ducts. To successfully repair the damage to the biliary epithelium, cholangiocytes must have the ability to proliferate and expand the ductular mass by forming branching tubular structures.12 During this process, hepatic progenitor cells generate “reactive” cholangiocytes; these are arranged into tubeless structures, and their phenotype has some features reminiscent of ductal plate cell. Reactive cholangiocytes are characterized by motile properties and active production of growth factors and cytokines, and are usually found at the periphery of the portal tract, close to parenchymal areas characterized by necro-inflammation and activation of the hepatic progenitor cell compartment.13–15 Ductular reaction is particularly evident in chronic cholestatic diseases.
Reactive ductules are able to release a vast array of paracrine signals, by which they establish an intensive cross-talk with multiple cell types, needed to sustain the intense remodelling activity characteristic of biliary repair. The “dark side” of these mechanisms is the generation of an exaggerated neo-angiogenic response with production of new fibro-vascular stroma and progressive liver fibrosis.8, 13 The close association of structural changes of the biliary epithelium with adaptive vascular responses has been well documented in experimental models of liver damage. For example, in bile duct ligation (BDL),3, 10 and αnaphthylisotiocyanate (ANIT) treatment,16 selective cholangiocyte proliferation is associated to a marked proliferation of the PBP. Furthermore, in genetic and neoplastic cholangiopathies cholangiocytes secrete and respond to angiogenic factors in a way that is reminiscent of their foetal behaviour. In these conditions, bile duct growth is paralleled by the reactivation of rich angiogenic functions, necessary to support the vascularization of the cystic epithelium in congenital ciliary protein defects,17–20 and the generation of the tumour reactive stroma in cholangiocarcinoma (CCA).21, 22
3.1. Chronic liver disease
Progression of chronic liver diseases (CLDs) is driven by persistent and extensive necro-inflammatory processes associated with fibrogenesis, as observed in viral hepatitis and alcoholic liver disease (ALD). In most cases, CLDs progression culminates in the end-point of cirrhosis. Irrespective of the aetiology, angiogenesis is an important mechanism underpinning the fibrogenic process, since evolution to cirrhosis is strictly associated with abnormal vascular remodelling.23, 24
CLDs featuring septal fibrosis, chronic viral hepatitis (HCV, HBV) are a good model to highlight the relationship between angiogenesis and the extent of fibrosis. Intense vascular remodelling has been documented at the interface hepatitis in HCV patients, where it strongly correlated with the degree of inflammation and fibrosis.25 Massive neo-angiogenesis is the result of the up-regulation of several angiogenic factors released by several cell types activated in the chronic wound healing process (ductular reactive cells, hepatocytes, portal myofibroblasts, macrophages), as well as by the morpho-structural modifications occurring with the development of bridging fibrosis, leading to tissue hypoxia. In ALD, the most common form of CLD worldwide,26 chronic Et-OH consumption is an important inducer of liver tissue hypoxia, among other pathogenic mechanisms contributing to liver damage. In a rat model of ALD, local low levels of tissue oxygen have been documented in periportal and centrilobular areas concurrently with peaks of blood Et-OH levels. Angiogenic growth factors, such as the vascular endothelial growth factor (VEGF) and the platelet-derived growth factor (PDGF), are markedly up-regulated in response to hypoxia.27 Recent data indicate that in rats, chronic Et-OH administration is associated with expression of angiogenic factors and consequent neoangiogenesis.28 However, the nature of the molecular factors mediating this process is still unclear. Preliminary data from our lab show that in ALD, ductular reactive cells express VEGFR-2 (see Figure 2 D). Et-OH administration in mice is also associated with Sonic Hedgehog (Hh)-induced proliferation of ductular cells, and lobular accumulation of Hh-responsive progenitors correlates with disease severity in ALD patients.29 Notably, activation of the progenitor cell compartment yields a strong prognostic significance in ALD, in fact it closely correlates with severity of liver disease and with short-term mortality in acute hepatitis.30 Overall, these data support the clinical relevance of morphogenetic signaling in mediated hepatic progenitor cells activation in Et-OH liver damage and other CLDs (see below).31
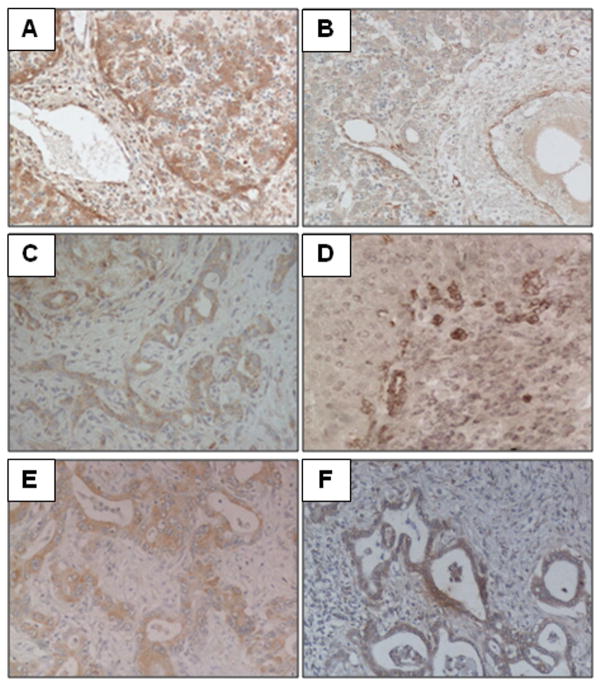
Figure 2. Immunohistochemical expression of VEGFR-2 in liver development and disease.
As bile ducts develop, ductal plate cells transiently express VEGFR-2 (A), until a lumen is formed and the nascent bile duct progressively migrates into the portal space (B). Reactive cholangiocytes display a de novo expression of VEGFR-2 during ductular reaction, as shown in samples of biliary atresia (C) and ALD (D), as well as neoplastic bile ducts (E – CCA). In polycystic liver diseases (F – Caroli disease) the cystic epithelium shows a marked upregulation of VEGFR-2 which, along with other angiogenic signals, is responsible for cysts enlargement. (200x magnification).
4. Cholangiocarcinoma (CCA)
Angiogenesis is a fundamental mechanism underlying tumour growth and invasion. The extent of neovascularization in CCA, is generally less developed than in hepatocellular carcinoma,32 however, recent studies suggest a role for VEGF in CCA proliferation. CCA expresses VEGF and VEGF receptors, which promote tumour growth and neoangiogenesis by mediating the effects of estrogens on tumour cells.21, 22 In extrahepatic CCA, the high tumour vascularization correlated with poor prognosis, but not with VEGF-A expression,33 indicating the involvement of other angiogenic pathways. Another crucial event in CCA invasiveness is tumour lymphangiogenesis, which is responsible for the early metastatisation to the regional lymph nodes, a critical step limiting curative treatments. Abundance in lymphatic vascular structures is observed in most CCA, in which the lymphatic spread is closely related to VEGF-C expression.34 Indeed, it is tempting to speculate that VEGF-C secreted by cancer and associated stromal cells plays a major role in lymphangiogenesis, stimulating the growth and the dilatation of peritumoral lymphatic vessels. The role of PDGF will be discussed below.
5. Molecular mechanisms regulating vascular functions in cholangiocytes
a) Vascular endothelial growth factors (VEGF) and Angiopoietins
Cholangiocytes produce and respond to a variety of vascular molecules enabling them to cross-talk with endothelial cells (ECs) and pericytes, the fundamental cell components of vascular structures. VEGF is the most important driver of vasculogenesis, the process by which ECs proliferate and differentiate within a previously avascular tissue, giving rise to new blood vessels. Angiogenesis, the remodelling process by which newly formed vessels sprout to sustain the maturation and stabilization of the vascular network, is instead regulated by the angiopoietins.35 VEGF is a member of a family of related growth factors that includes VEGF-A, -B, -C, -D and -E and placenta growth factor. VEGF interacts with three tyrosine-kinase receptors, VEGFR-1 (Flt-1), VEGFR-2 (Flk-1/KDR) and VEGFR-3.35 Hematopoietic stem cells, ECs and vascular smooth muscle cells express VEGF and its receptors; their presence has also been recently documented in ductal plate cells, cholangiocytes and even in hepatic stellate cells (HSCs).36
Angiopoietins (Ang-1 and Ang-2) bind to the tyrosine kinase receptor Tie-2 expressed by ECs along with the VEGF receptors.37 Angiopoietins have opposite effects on their receptor: Ang-1 activates Tie-2 by tyrosine phosphorylation, while Ang-2 antagonizes the Ang-1/Tie-2 binding; therefore, Tie-2 activation is determined by the relative balance between Ang-1 and Ang-2.
During development, VEGF is the signal linking bile duct morphogenesis and liver vascularization. We have previously reported that ductal plate cells produce a VEGF gradient that increases as bile ducts develop and cooperates with the angiopoietins system. VEGF-A produced by cholangiocytes acts on ECs and their precursors to promote arterial and PBP vasculogenesis, while hepatoblasts produce Ang-1 that induces the maturation of the nascent arterial vessels by recruiting mural pericytes.11
Cholangiocytes regain the ability to produce and respond to VEGF in disease conditions (Figure 2). In the BDL model, cholangiocytes increase the production of VEGF-A and VEGF-C, to promote bile ducts proliferation via a paracrine/autocrine mechanism.3 Most likely, VEGF represents a protective mechanism that drives the adaptive/reparative response in experimental obstructive cholestasis. In an experimental model of ischemic injury (hepatic artery ligation), the progressive disappearance of the PBP was paralleled by a decreased cholangiocytes proliferation and VEGF-A expression, along with increased bile duct apoptosis. These effects were counteracted by administration of VEGF, suggesting a fundamental role of VEGF in both cholangiocyte and PBP proliferation.38 On the other hand, VEGF is involved in the pathological angiogenesis occurring in the progression of chronic liver diseases where it is associated with the development of septal fibrosis and therefore, with the progression to cirrhosis.39 Activated HSCs, the main actors in fibrosis progression, secrete Ang-1 thus favouring angiogenesis.40 In primary biliary cirrhosis (PBC) the increased expression of VEGF-A, Ang-1, Ang-2 and Tie-2 receptor by ECs and periportal hepatocytes is responsible for the brisk angiogenesis occurring in close proximity to the damaged bile ducts,41 which may contribute to inflammatory cells recruitment, worsening the disease. Notably, a strong reduction in portal fibrosis and portal hypertension was obtained in BDL rats by inhibiting VEGFR-2.42
Recent studies from our laboratory have highlighted the critical involvement of VEGF signaling in the pathogenesis of Polycystic Liver Disease associated to Autosomal Dominant Polycystic Kidney Disease (PLD-ADPKD). PLD-ADPKD is a genetically transmitted disease caused by mutations in the PKD1 or PKD2 genes, encoding for polycistin-1 (PC1) and polycystin-2 (PC2), respectively.43 PC1 and PC2 are transmembrane proteins expressed in the primary cilium of renal tubular and bile duct epithelial cells, which regulate signaling pathways involved in ductal morphogenesis, proliferation and differentiation. When PCs are defective, cholangiocytes maintain an immature proliferative phenotype, responsible for the formation of multiple, large cysts spanning the liver parenchyma. Liver cysts are extensively surrounded by a rich vascular bed which lays in close proximity to them. The signals linking PCs deficiency to cystogenesis and disease progression have been recently started to be clarified.44 Our group has previously reported a marked upregulation of VEGF together with its receptors VEGFR-1 and VEGFR-2, and of Ang-1 in conjunction with its cognate receptor Tie-2 in the epithelium layering the liver cysts. VEGF stimulated cystic vascularization as well as cholangiocyte proliferation with a dose-dependent effect.17 Further studies demonstrated that an overactive MEK/ERK1/2/mTOR pathway is responsible for hypoxia-inducible factor1α-dependent VEGF production by cystic cholangiocytes, stimulating autocrine growth of cysts via VEGFR-2.19, 20 The observation that VEGF mediates the progressive enlargement of liver cysts, has laid the foundations to the pharmacological interference with angiogenic signaling to inhibit disease progression using VEGFR-2 or mTOR inhibitors.19 However, a paradoxical dose-dependent effect of Sorafenib, an oral inhibitor of Ras-1 signaling, has been shown in a mouse model of PC2 defects,45 where it results in a further increased MEK/ERK signaling and consequently, in the progression of cysts enlargement.
b) Platelet-derived growth factor (PDGF)
PDGF is a family of four polypeptide chains (A, B, C, D) assembling in four homodimers (AA, BB, CC, DD) and one heterodimer (AB). PDGF ligands specifically bind to two different tyrosine kinase receptors: PDGFRα binds PDGF-A, -B and -C, while PDGF-B and -D act on PDGFRβ. PDGF is very important for HSCs function, and has a relevant role in the fibrogenetic process during biliary repair. PDGF effects on HSCs range from proliferation, to migration and transdifferentiation into myofibroblasts (MFs).46, 47 After induction of experimental cholestasis (BDL), reactive cholangiocytes express increasing amounts of PDGF-B,48 which in turn act on PDGFRβ-expressing HSCs, stimulating their chemotaxis towards injured ducts,49 and their proliferation before transdifferentiation into MFs.50 Moreover, PDGF-B regulates both HSCs,51 and cholangiocytes,52 expansion following biliary injury by activating Hedgehog signaling in both cell types. In addition to fibrogenesis, PDGF is actively involved in the regulation of hepatic vasculogenesis. PDGF-B stimulates HSCs to acquire an angiogenic phenotype similar to pericytes, thus promoting vascular tube formation and HSCs coverage of sinusoids.53 PDGF also mediates the cross-talk between ECs and pericytes: ECs stimulate pericytes recruitment through PDGFRβ, to stabilize the endothelium in the developing vasculature, through a PDGF-B and an ephrin-dependent mechanism.53–55
PDGF is emerging as an important signaling pathway in CCA, where it modulates the interactions between the epithelial and the stromal components, which is particularly abundant in CCA. Among the different cell elements populating the tumour reactive stroma in CCA, activated fibroblasts, also termed as cancer-associated fibroblasts (CAFs) are the most important. CAFs provide cancer cells with a number of paracrine signals, favouring tumour proliferation, survival and growth. Overexpression of PDGFRβ in the stromal compartment of CCA is related to the most significant “network connectivity” with the tumoural compartment.56 On the other hand, CAF-derived PDGF-B protects CCA cells from death induced by tumour necrosis factor-related apoptosis-inducing ligand (TRAIL) in a Hh signaling dependent-manner (see below).57
Recent data from our laboratory reveal a role for PDGF signaling also in the generation of the stromal microenvironment, which is a crucial step in CCA cancerogenesis. The paracrine effects of PDGF secreted by CCA cholangiocytes promote the recruitment of CAFs into the CCA microenvironment. PDGF-D, specifically secreted by CCA cholangiocytes, binds to PDGFRβ and recruits resident fibroblasts to the sites of neoplastic growth acting via the Rho GTPases, critical mediators of cell migration. Pharmacological targeting of tumour/stroma interactions using PDGF inhibitors may therefore offer a novel molecularly targeted therapeutic approach in CCA. (Cadamuro M, submitted)
c) Morphogens
Notch is a highly conserved pathway mainly involved in cell fate determination during organogenesis. This peculiar signaling requires cell-cell contact since both receptors (Notch1-4) and ligands (Jagged-1 and -2, Delta-like-1, -3 and -4) are transmembrane proteins. Notch receptors expressed on the “receiving cells” are activated by the binding with ligands, thus leading to a downstream signaling that culminates with the activation of RBP-Jκ transcription factor and the expression of target genes Hes-1, Hes-5 and Hey-1.58 In the liver, the activation of Notch signaling in the hepatoblast induces the expression of the biliary-specific transcription factors Hnf1β and Hnf6, drivers of the commitment toward the biliary lineage. During development, Notch-2 is expressed in biliary epithelial cells adjacent to the Jagged-1 positive mesenchymal cells.59, 60 Mutations in the genes encoding for Jagged-1,61, 62 (and more rarely Notch2,63) cause Alagille syndrome (AGS), a cholestatic cholangiopathy characterized by ductopenia along with defective branching of the biliary tree and vascular malformations. Experimental models of Notch selective defects in hepatoblasts/cholangiocytes result in bile duct paucity, resembling AGS features.64, 65 Moreover, Jagged-1 inactivation in portal vein mesenchymal cells alters bile ducts morphogenesis and impairs the formation of both portal mesenchyme and artery vascularization, a phenotype shown also in Hnf1β and Hnf6 KO mice.66 In addition to the role played in liver development, Notch is a modulator of intrahepatic bile ducts regulation also in adult liver in disease conditions. After biliary damage, Notch signaling plays a fundamental role in the activation of hepatic progenitor cells,67 and in ductal morphogenesis. The proper biliary repair requires the coordinated and differential action of both Notch-1 and Notch-2. If only Notch-2 is defective, the de novo tubule formation is defective. Using genetic models of Notch loss of function, a specific involvement of Notch-2 in tubular morphogenesis, rather than in biliary commitment of hepatic progenitor cells has been documented. (Fiorotto R, submitted)
Another morphogenetic pathway strongly involved in angiogenic processes is the Hh. Hh signaling is typical of stromal and progenitor cells, whilst it is not active in mature cholangiocytes. In disease conditions, i.e. liver fibrosis, there is an up-regulation of the Hh system in both epithelial and stromal cells.68 In BLD animals, PDGF stimulates the release of Hh containing microparticles by HSCs and cholangiocytes, altering gene expression in Hh-responsive ECs thus favouring vasculogenesis in the fibrotic tissue.69 In CCA, Hh signaling mediates cytoprotective effects of PDGF-B released from CAFs against TRAIL-induced apoptosis.57 Therefore, inhibition of Hh signaling may provide a novel tool to induce apoptosis in CCA cells.
5. Conclusions
Angiogenic factors have emerged as important mediators of cell functions in the biliary epithelium. They exert both autocrine functions and paracrine effects, orchestrating an extensive cross-talk between cholangiocytes and multiple cell types, including ECs and pericytes. Epithelial angiogenic signaling plays an important role in liver development as well as in liver repair, congenital cholangiopathies and biliary carcinogenesis. Therapeutic interference with angiogenic signals has shown promises in several liver diseases, including developmental and neoplastic cholangiopathies and is an area that deserves further investigation.
Acknowledgments
Financial Support
This work was supported by NIH grant DK079005, by a NIH Grant DK34989: Silvio O. Conte Digestive Diseases Research Core Centers, by PRIN 2009ARYX4T_005, by CARIPLO 2011-0470 to M.S. and by a grant from Fondazione Telethon, GGP09-189 and by Progetto di Ricerca Ateneo 2011, CPD 113799/11 to L.F.
Footnotes
Conflict of interest: the authors have nothing to disclose.
References
- 1.Strazzabosco M, Fabris L. Functional anatomy of normal bile ducts. Anat Rec (Hoboken) 2008;291:653–60. doi: 10.1002/ar.20664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alpini G, Roberts S, Kuntz SM, et al. Morphological, molecular, and functional heterogeneity of cholangiocytes from normal rat liver. Gastroenterology. 1996;110:1636–43. doi: 10.1053/gast.1996.v110.pm8613073. [DOI] [PubMed] [Google Scholar]
- 3.Gaudio E, Onori P, Pannarale L, Alvaro D. Hepatic microcirculation and peribiliary plexus in experimental biliary cirrhosis: a morphological study. Gastroenterology. 1996;111:1118–24. doi: 10.1016/s0016-5085(96)70081-1. [DOI] [PubMed] [Google Scholar]
- 4.Lemaigre FP. Development of the biliary tract. Mech Dev. 2003;120:81–7. doi: 10.1016/s0925-4773(02)00334-9. [DOI] [PubMed] [Google Scholar]
- 5.Lemaigre FP. Mechanisms of liver development: concepts for understanding liver disorders and design of novel therapies. Gastroenterology. 2009;137:62–79. doi: 10.1053/j.gastro.2009.03.035. [DOI] [PubMed] [Google Scholar]
- 6.Roskams T, Desmet V. Embryology of extra- and intrahepatic bile ducts, the ductal plate. Anat Rec (Hoboken) 2008;291:628–35. doi: 10.1002/ar.20710. [DOI] [PubMed] [Google Scholar]
- 7.Terada T, Nakanuma Y. Development of human peribiliary capillary plexus: a lectin-histochemical and immunohistochemical study. Hepatology. 1993;18:529–36. [PubMed] [Google Scholar]
- 8.Desmet V, Roskams T, Van Eyken P. Ductular reaction in the liver. Pathol Res Pract. 1995;191:513–24. doi: 10.1016/s0344-0338(11)80870-8. [DOI] [PubMed] [Google Scholar]
- 9.Desmet VJ. Congenital diseases of intrahepatic bile ducts: variations on the theme “ductal plate malformation”. Hepatology. 1992;16:1069–83. doi: 10.1002/hep.1840160434. [DOI] [PubMed] [Google Scholar]
- 10.Clotman F, Libbrecht L, Gresh L, et al. Hepatic artery malformations associated with a primary defect in intrahepatic bile duct development. J Hepatol. 2003;39:686–92. doi: 10.1016/s0168-8278(03)00409-4. [DOI] [PubMed] [Google Scholar]
- 11.Fabris L, Cadamuro M, Libbrecht L, et al. Epithelial expression of angiogenic growth factors modulate arterial vasculogenesis in human liver development. Hepatology. 2008;47:719–28. doi: 10.1002/hep.22015. [DOI] [PubMed] [Google Scholar]
- 12.Fausto N. Liver regeneration and repair: hepatocytes, progenitor cells, and stem cells. Hepatology. 2004;39:1477–87. doi: 10.1002/hep.20214. [DOI] [PubMed] [Google Scholar]
- 13.Lazaridis KN, Strazzabosco M, Larusso NF. The cholangiopathies: disorders of biliary epithelia. Gastroenterology. 2004;127:1565–77. doi: 10.1053/j.gastro.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 14.Strazzabosco M, Fabris L, Spirli C. Pathophysiology of cholangiopathies. J Clin Gastroenterol. 2005;39:S90–S102. doi: 10.1097/01.mcg.0000155549.29643.ad. [DOI] [PubMed] [Google Scholar]
- 15.Fabris L, Strazzabosco M. Epithelial-mesenchymal interactions in biliary diseases. Semin Liver Dis. 2011;31:11–32. doi: 10.1055/s-0031-1272832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Masyuk TV, Ritman EL, LaRusso NF. Hepatic artery and portal vein remodeling in rat liver: vascular response to selective cholangiocyte proliferation. Am J Pathol. 2003;162:1175–82. doi: 10.1016/S0002-9440(10)63913-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fabris L, Cadamuro M, Fiorotto R, et al. Effects of angiogenic factor overexpression by human and rodent cholangiocytes in polycystic liver diseases. Hepatology. 2006;43:1001–12. doi: 10.1002/hep.21143. [DOI] [PubMed] [Google Scholar]
- 18.Fabris L, Strazzabosco M, Crosby HA, et al. Characterization and isolation of ductular cells coexpressing neural cell adhesion molecule and Bcl-2 from primary cholangiopathies and ductal plate malformations. Am J Pathol. 2000;156:1599–612. doi: 10.1016/S0002-9440(10)65032-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spirli C, Okolicsanyi S, Fiorotto R, et al. Mammalian target of rapamycin regulates vascular endothelial growth factor-dependent liver cyst growth in polycystin-2-defective mice. Hepatology. 2010;51:1778–88. doi: 10.1002/hep.23511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spirli C, Okolicsanyi S, Fiorotto R, et al. ERK1/2-dependent vascular endothelial growth factor signaling sustains cyst growth in polycystin-2 defective mice. Gastroenterology. 2010;138:360–71. e7. doi: 10.1053/j.gastro.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alvaro D, Barbaro B, Franchitto A, et al. Estrogens and insulin-like growth factor 1 modulate neoplastic cell growth in human cholangiocarcinoma. Am J Pathol. 2006;169:877–88. doi: 10.2353/ajpath.2006.050464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mancino A, Mancino MG, Glaser SS, et al. Estrogens stimulate the proliferation of human cholangiocarcinoma by inducing the expression and secretion of vascular endothelial growth factor. Dig Liver Dis. 2009;41:156–63. doi: 10.1016/j.dld.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Medina J, Arroyo AG, Sanchez-Madrid F, Moreno-Otero R. Angiogenesis in chronic inflammatory liver disease. Hepatology. 2004;39:1185–95. doi: 10.1002/hep.20193. [DOI] [PubMed] [Google Scholar]
- 24.Pinzani M, Rombouts K. Liver fibrosis: from the bench to clinical targets. Dig Liver Dis. 2004;36:231–42. doi: 10.1016/j.dld.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 25.Gabriel A, Kukla M, Wilk M, Liszka L, Petelenz M, Musialik J. Angiogenesis in chronic hepatitis C is associated with inflammatory activity grade and fibrosis stage. Pathol Res Pract. 2009;205:758–64. doi: 10.1016/j.prp.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 26.Mann RE, Smart RG, Govoni R. The epidemiology of alcoholic liver disease. Alcohol Res Health. 2003;27:209–19. [PMC free article] [PubMed] [Google Scholar]
- 27.Bardag-Gorce F, French BA, Li J, et al. The importance of cycling of blood alcohol levels in the pathogenesis of experimental alcoholic liver disease in rats. Gastroenterology. 2002;123:325–35. doi: 10.1053/gast.2002.34177. [DOI] [PubMed] [Google Scholar]
- 28.Das SK, Mukherjee S, Vasudevan DM. Effects of long term ethanol consumption mediated oxidative stress on neovessel generation in liver. Toxicol Mech Methods. 2012;22:375–82. doi: 10.3109/15376516.2012.666651. [DOI] [PubMed] [Google Scholar]
- 29.Jung Y, Brown KD, Witek RP, et al. Accumulation of hedgehog-responsive progenitors parallels alcoholic liver disease severity in mice and humans. Gastroenterology. 2008;134:1532–43. doi: 10.1053/j.gastro.2008.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sancho-Bru P, Altamirano J, Rodrigo-Torres D, et al. Liver progenitor cell markers correlate with liver damage and predict short-term mortality in patients with alcoholic hepatitis. Hepatology. 2012;55:1931–41. doi: 10.1002/hep.25614. [DOI] [PubMed] [Google Scholar]
- 31.de Pereira TA, Witek RP, Syn WK, et al. Viral factors induce Hedgehog pathway activation in humans with viral hepatitis, cirrhosis, and hepatocellular carcinoma. Lab Invest. 2010;90:1690–703. doi: 10.1038/labinvest.2010.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Okuda K, Nakanuma Y, Miyazaki M. Cholangiocarcinoma: recent progress. Part 2: molecular pathology and treatment. J Gastroenterol Hepatol. 2002;17:1056–63. doi: 10.1046/j.1440-1746.2002.02780.x. [DOI] [PubMed] [Google Scholar]
- 33.Mobius C, Demuth C, Aigner T, et al. Evaluation of VEGF A expression and microvascular density as prognostic factors in extrahepatic cholangiocarcinoma. Eur J Surg Oncol. 2007;33:1025–9. doi: 10.1016/j.ejso.2007.02.020. [DOI] [PubMed] [Google Scholar]
- 34.Aishima S, Nishihara Y, Iguchi T, et al. Lymphatic spread is related to VEGF-C expression and D2-40-positive myofibroblasts in intrahepatic cholangiocarcinoma. Mod Pathol. 2008;21:256–64. doi: 10.1038/modpathol.3800985. [DOI] [PubMed] [Google Scholar]
- 35.Roy H, Bhardwaj S, Yla-Herttuala S. Biology of vascular endothelial growth factors. FEBS Lett. 2006;580:2879–87. doi: 10.1016/j.febslet.2006.03.087. [DOI] [PubMed] [Google Scholar]
- 36.Gaudio E, Franchitto A, Pannarale L, et al. Cholangiocytes and blood supply. World J Gastroenterol. 2006;12:3546–52. doi: 10.3748/wjg.v12.i22.3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morisada T, Kubota Y, Urano T, Suda T, Oike Y. Angiopoietins and angiopoietin-like proteins in angiogenesis. Endothelium. 2006;13:71–9. doi: 10.1080/10623320600697989. [DOI] [PubMed] [Google Scholar]
- 38.Gaudio E, Barbaro B, Alvaro D, et al. Administration of r-VEGF-A prevents hepatic artery ligation-induced bile duct damage in bile duct ligated rats. Am J Physiol Gastrointest Liver Physiol. 2006;291:G307–17. doi: 10.1152/ajpgi.00507.2005. [DOI] [PubMed] [Google Scholar]
- 39.Fernandez M, Semela D, Bruix J, Colle I, Pinzani M, Bosch J. Angiogenesis in liver disease. J Hepatol. 2009;50:604–20. doi: 10.1016/j.jhep.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 40.Taura K, De Minicis S, Seki E, et al. Hepatic stellate cells secrete angiopoietin 1 that induces angiogenesis in liver fibrosis. Gastroenterology. 2008;135:1729–38. doi: 10.1053/j.gastro.2008.07.065. [DOI] [PubMed] [Google Scholar]
- 41.Medina J, Sanz-Cameno P, Garcia-Buey L, Martin-Vilchez S, Lopez-Cabrera M, Moreno-Otero R. Evidence of angiogenesis in primary biliary cirrhosis: an immunohistochemical descriptive study. J Hepatol. 2005;42:124–31. doi: 10.1016/j.jhep.2004.09.024. [DOI] [PubMed] [Google Scholar]
- 42.Mejias M, Garcia-Pras E, Tiani C, Miquel R, Bosch J, Fernandez M. Beneficial effects of sorafenib on splanchnic, intrahepatic, and portocollateral circulations in portal hypertensive and cirrhotic rats. Hepatology. 2009;49:1245–56. doi: 10.1002/hep.22758. [DOI] [PubMed] [Google Scholar]
- 43.Igarashi P. Overview: nonmammalian organisms for studies of kidney development and disease. J Am Soc Nephrol. 2005;16:296–8. doi: 10.1681/ASN.2004110951. [DOI] [PubMed] [Google Scholar]
- 44.Praetorius HA, Spring KR. A physiological view of the primary cilium. Annu Rev Physiol. 2005;67:515–29. doi: 10.1146/annurev.physiol.67.040403.101353. [DOI] [PubMed] [Google Scholar]
- 45.Spirli C, Morell CM, Locatelli L, et al. Cyclic AMP/PKA-dependent paradoxical activation of Raf/MEK/ERK signaling in polycystin-2 defective mice treated with Sorafenib. Hepatology. 2012 doi: 10.1002/hep.25872. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 46.Friedman SL, Arthur MJ. Activation of cultured rat hepatic lipocytes by Kupffer cell conditioned medium. Direct enhancement of matrix synthesis and stimulation of cell proliferation via induction of platelet-derived growth factor receptors. J Clin Invest. 1989;84:1780–5. doi: 10.1172/JCI114362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pinzani M, Gesualdo L, Sabbah GM, Abboud HE. Effects of platelet-derived growth factor and other polypeptide mitogens on DNA synthesis and growth of cultured rat liver fat-storing cells. J Clin Invest. 1989;84:1786–93. doi: 10.1172/JCI114363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grappone C, Pinzani M, Parola M, et al. Expression of platelet-derived growth factor in newly formed cholangiocytes during experimental biliary fibrosis in rats. J Hepatol. 1999;31:100–9. doi: 10.1016/s0168-8278(99)80169-x. [DOI] [PubMed] [Google Scholar]
- 49.Kinnman N, Hultcrantz R, Barbu V, et al. PDGF-mediated chemoattraction of hepatic stellate cells by bile duct segments in cholestatic liver injury. Lab Invest. 2000;80:697–707. doi: 10.1038/labinvest.3780073. [DOI] [PubMed] [Google Scholar]
- 50.Kinnman N, Goria O, Wendum D, et al. Hepatic stellate cell proliferation is an early platelet-derived growth factor-mediated cellular event in rat cholestatic liver injury. Lab Invest. 2001;81:1709–16. doi: 10.1038/labinvest.3780384. [DOI] [PubMed] [Google Scholar]
- 51.Yang L, Wang Y, Mao H, et al. Sonic hedgehog is an autocrine viability factor for myofibroblastic hepatic stellate cells. J Hepatol. 2008;48:98–106. doi: 10.1016/j.jhep.2007.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Omenetti A, Popov Y, Jung Y, et al. The hedgehog pathway regulates remodelling responses to biliary obstruction in rats. Gut. 2008;57:1275–82. doi: 10.1136/gut.2008.148619. [DOI] [PubMed] [Google Scholar]
- 53.Semela D, Das A, Langer D, Kang N, Leof E, Shah V. Platelet-derived growth factor signaling through ephrin-b2 regulates hepatic vascular structure and function. Gastroenterology. 2008;135:671–9. doi: 10.1053/j.gastro.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hellstrom M, Kalen M, Lindahl P, Abramsson A, Betsholtz C. Role of PDGF-B and PDGFR-beta in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development. 1999;126:3047–55. doi: 10.1242/dev.126.14.3047. [DOI] [PubMed] [Google Scholar]
- 55.Lindahl P, Johansson BR, Leveen P, Betsholtz C. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science. 1997;277:242–5. doi: 10.1126/science.277.5323.242. [DOI] [PubMed] [Google Scholar]
- 56.Andersen JB, Spee B, Blechacz BR, et al. Genomic and genetic characterization of cholangiocarcinoma identifies therapeutic targets for tyrosine kinase inhibitors. Gastroenterology. 2012;142:1021–31. e15. doi: 10.1053/j.gastro.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fingas CD, Bronk SF, Werneburg NW, et al. Myofibroblast-derived PDGF-BB promotes Hedgehog survival signaling in cholangiocarcinoma cells. Hepatology. 2011;54:2076–88. doi: 10.1002/hep.24588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lai EC. Notch signaling: control of cell communication and cell fate. Development. 2004;131:965–73. doi: 10.1242/dev.01074. [DOI] [PubMed] [Google Scholar]
- 59.Zong Y, Panikkar A, Xu J, et al. Notch signaling controls liver development by regulating biliary differentiation. Development. 2009;136:1727–39. doi: 10.1242/dev.029140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tanimizu N, Miyajima A. Notch signaling controls hepatoblast differentiation by altering the expression of liver-enriched transcription factors. J Cell Sci. 2004;117:3165–74. doi: 10.1242/jcs.01169. [DOI] [PubMed] [Google Scholar]
- 61.Li L, Krantz ID, Deng Y, et al. Alagille syndrome is caused by mutations in human Jagged1, which encodes a ligand for Notch1. Nat Genet. 1997;16:243–51. doi: 10.1038/ng0797-243. [DOI] [PubMed] [Google Scholar]
- 62.Oda T, Elkahloun AG, Pike BL, et al. Mutations in the human Jagged1 gene are responsible for Alagille syndrome. Nat Genet. 1997;16:235–42. doi: 10.1038/ng0797-235. [DOI] [PubMed] [Google Scholar]
- 63.McDaniell R, Warthen DM, Sanchez-Lara PA, et al. NOTCH2 mutations cause Alagille syndrome, a heterogeneous disorder of the notch signaling pathway. Am J Hum Genet. 2006;79:169–73. doi: 10.1086/505332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lozier J, McCright B, Gridley T. Notch signaling regulates bile duct morphogenesis in mice. PLoS One. 2008;3:e1851. doi: 10.1371/journal.pone.0001851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sparks EE, Huppert KA, Brown MA, Washington MK, Huppert SS. Notch signaling regulates formation of the three-dimensional architecture of intrahepatic bile ducts in mice. Hepatology. 2010;51:1391–400. doi: 10.1002/hep.23431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hofmann JJ, Zovein AC, Koh H, Radtke F, Weinmaster G, Iruela-Arispe ML. Jagged1 in the portal vein mesenchyme regulates intrahepatic bile duct development: insights into Alagille syndrome. Development. 2011;137:4061–72. doi: 10.1242/dev.052118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Boulter L, Govaere O, Bird TG, et al. Macrophage-derived Wnt opposes Notch signaling to specify hepatic progenitor cell fate in chronic liver disease. Nat Med. 2012;18:572–9. doi: 10.1038/nm.2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Omenetti A, Porrello A, Jung Y, et al. Hedgehog signaling regulates epithelial-mesenchymal transition during biliary fibrosis in rodents and humans. J Clin Invest. 2008;118:3331–42. doi: 10.1172/JCI35875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Witek RP, Yang L, Liu R, et al. Liver cell-derived microparticles activate hedgehog signaling and alter gene expression in hepatic endothelial cells. Gastroenterology. 2009;136:320–30. e2. doi: 10.1053/j.gastro.2008.09.066. [DOI] [PMC free article] [PubMed] [Google Scholar]