Abstract
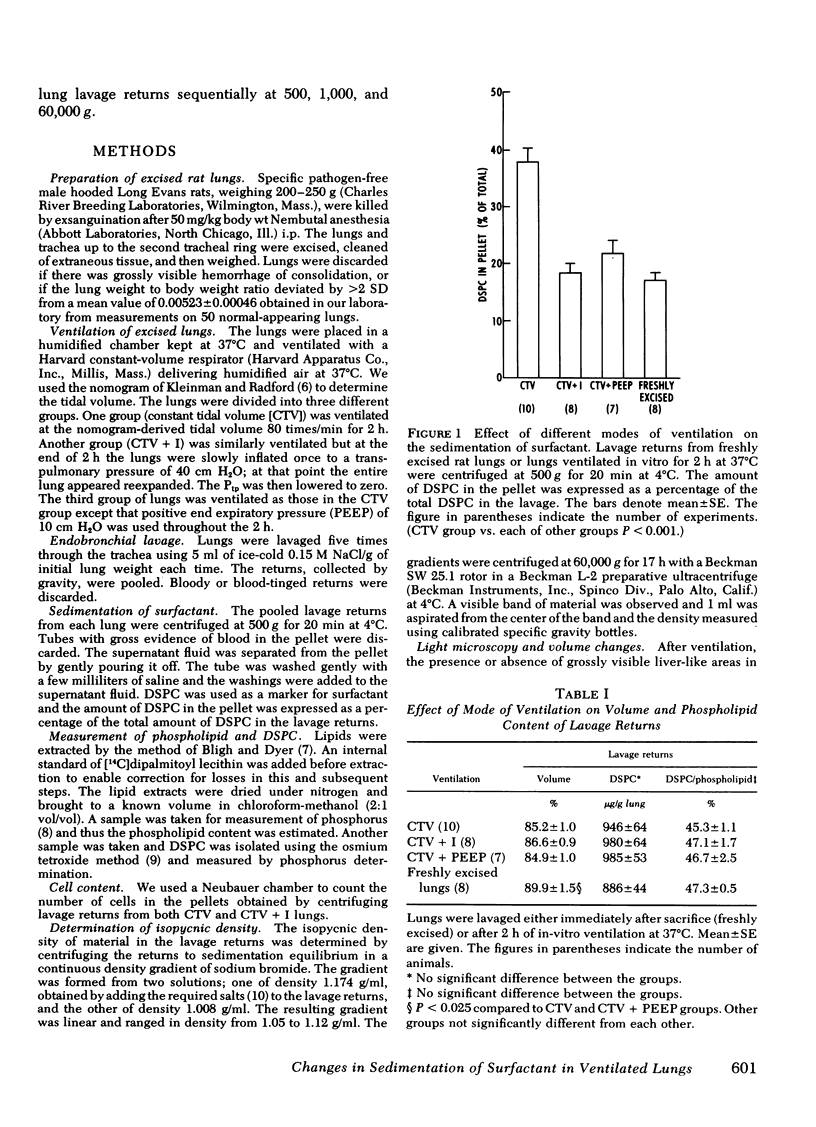
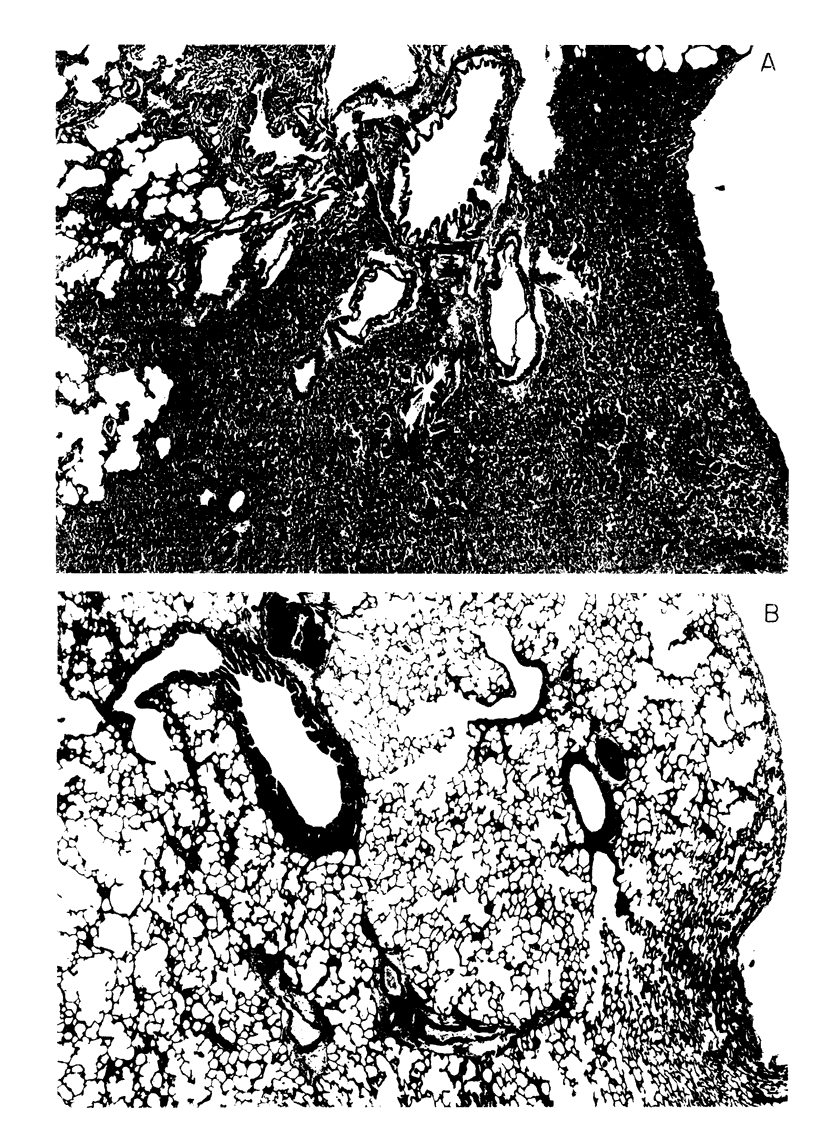
We ventilated excised rat lungs at a constant tidal volume (CTV); they developed areas of atelectasis which could be reversed by a large inflation (CTV + I) or prevented by the addition of positive end-expiratory pressure to the CTV. To explore the possibility that these modes of ventilation led to changes in surfactant, we lavaged the lungs and centrifuged the returns at 500 g; we measured the amount of disaturated phosphatidylcholine (DSPC) in the resultant pellet and supernatant fluid as a marker for surfactant. We found 16.9±1.5 (mean±SE), 38.0±2.4, 18.3±1.6, and 21.7±2.3% of the total lavage DSPC, in the pellet from freshly excised, CTV, CTV + I, and positive end-expiratory pressure to the CTV lungs, respectively. The total amount of lavage DSPC was the same in all groups.
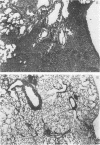
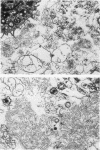
The ultrastructure of acellular material pelleted by sequential centrifugation of lavage returns at 500, 1,000, and 60,000 g was examined. We found mostly tubular myelin in the 500-g and 1,000-g pellets, but no tubular myelin in the 60,000-g pellet.
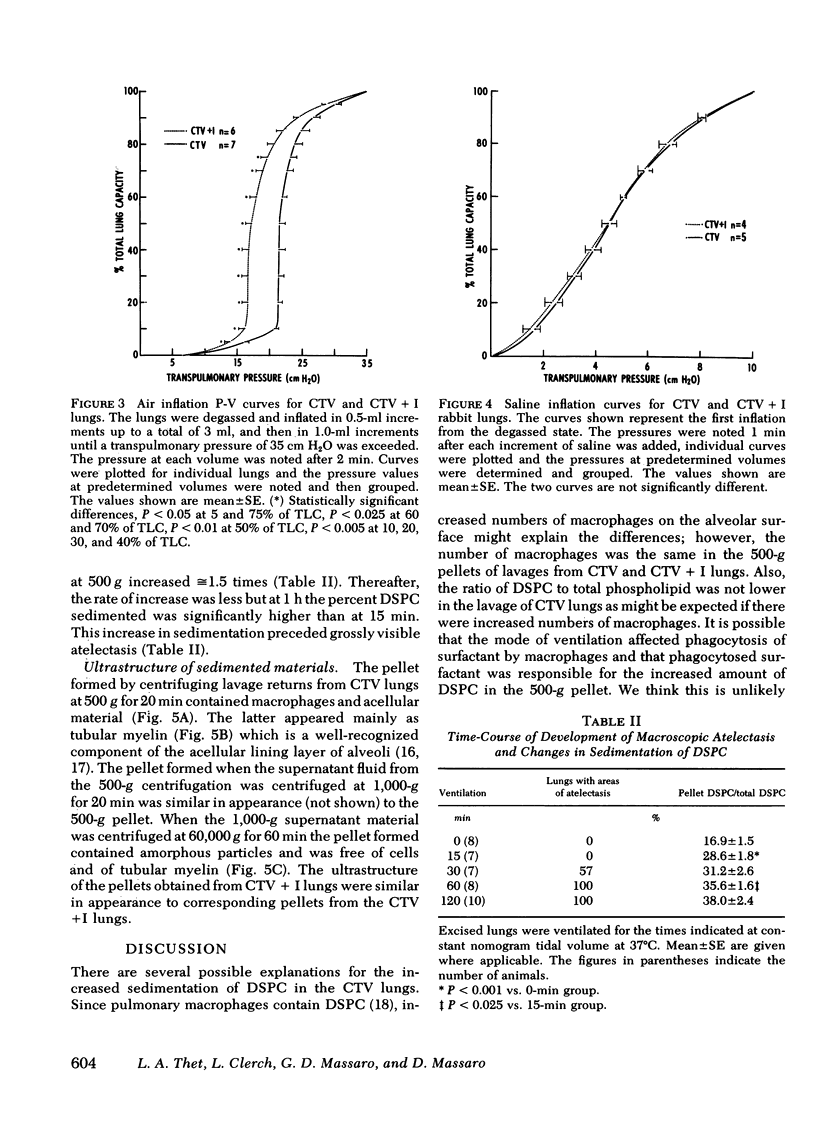
Air inflation pressure-volume measurements from the degassed state revealed that the opening pressure and recoil pressures up to 75% of total lung capacity were significantly higher in the CTV than in the CTV + I lungs. There were no differences between these groups in air deflation or in saline inflation and deflation pressure-volume measurements. Our findings suggest that CTV leads to increases in the tubular myelin form of surfactant and that this leads to increased surface tension in alveoli which results in alveolar collapse.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERNSTEIN L. The elastic pressure-volume curves of the lungs and thorax of the living rabbit. J Physiol. 1957 Oct 30;138(3):473–487. doi: 10.1113/jphysiol.1957.sp005864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Gail D. B., Massaro G. D., Massaro D. Influence of fasting on the lung. J Appl Physiol Respir Environ Exerc Physiol. 1977 Jan;42(1):88–92. doi: 10.1152/jappl.1977.42.1.88. [DOI] [PubMed] [Google Scholar]
- Gil J., Reiss O. K. Isolation and characterization of lamellar bodies and tubular myelin from rat lung homogenates. J Cell Biol. 1973 Jul;58(1):152–171. doi: 10.1083/jcb.58.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEDLEY-WHYTE J., LAVER M. B., BENDIXEN H. H. EFFECT OF CHANGES IN TIDAL VENTILATION ON PHYSIOLOGIC SHUNTING. Am J Physiol. 1964 Apr;206:891–897. doi: 10.1152/ajplegacy.1964.206.4.891. [DOI] [PubMed] [Google Scholar]
- Haldane J. S., Meakins J. C., Priestley J. G. The effects of shallow breathing. J Physiol. 1919 May 20;52(6):433–453. doi: 10.1113/jphysiol.1919.sp001842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KLEINMAN L. I., RADFORD E. P., Jr VENTILATION STANDARDS FOR SMALL MAMMALS. J Appl Physiol. 1964 Mar;19:360–362. doi: 10.1152/jappl.1964.19.2.360. [DOI] [PubMed] [Google Scholar]
- King R. J., Clements J. A. Surface active materials from dog lung. I. Method of isolation. Am J Physiol. 1972 Sep;223(3):707–714. doi: 10.1152/ajplegacy.1972.223.3.707. [DOI] [PubMed] [Google Scholar]
- MEAD J. Mechanical properties of lungs. Physiol Rev. 1961 Apr;41:281–330. doi: 10.1152/physrev.1961.41.2.281. [DOI] [PubMed] [Google Scholar]
- MEAD J., WHITTENBERGER J. L., RADFORD E. P., Jr Surface tension as a factor in pulmonary volume-pressure hysteresis. J Appl Physiol. 1957 Mar;10(2):191–196. doi: 10.1152/jappl.1957.10.2.191. [DOI] [PubMed] [Google Scholar]
- MORRISON W. R. A FAST, SIMPLE AND RELIABLE METHOD FOR THE MICRODETERMINATION OF PHOSPHORUS IN BIOLOGICAL MATERIALS. Anal Biochem. 1964 Feb;7:218–224. doi: 10.1016/0003-2697(64)90231-3. [DOI] [PubMed] [Google Scholar]
- Mason R. J., Huber G., Vaughan M. Synthesis of dipalmitoyl lecithin by alveolar macrophages. J Clin Invest. 1972 Jan;51(1):68–73. doi: 10.1172/JCI106798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason R. J., Nellenbogen J., Clements J. A. Isolation of disaturated phosphatidylcholine with osmium tetroxide. J Lipid Res. 1976 May;17(3):281–284. [PubMed] [Google Scholar]
- Massaro G. D., Massaro D. Hyperoxia: a stereologic ultrastructural examination of its influence on cytoplasmic components of the pulmonary granular pneumocyte. J Clin Invest. 1973 Mar;52(3):566–570. doi: 10.1172/JCI107217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielson D., Olsen D. B. The role of alveolar recruitment and de-recruitment in pressure-volume hysteresis in lungs. Respir Physiol. 1978 Jan;32(1):63–77. doi: 10.1016/0034-5687(78)90100-7. [DOI] [PubMed] [Google Scholar]
- STOECKENIUS W. An electron microscope study of myelin figures. J Biophys Biochem Cytol. 1959 May 25;5(3):491–500. doi: 10.1083/jcb.5.3.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherle W. A simple method for volumetry of organs in quantitative stereology. Mikroskopie. 1970 Jun;26(1):57–60. [PubMed] [Google Scholar]
- Untersee P., Gil J., Weibel E. R. Visualization of extracellular lining layer of lung alveoli by freeze-etching. Respir Physiol. 1971 Nov;13(2):171–185. doi: 10.1016/0034-5687(71)90088-0. [DOI] [PubMed] [Google Scholar]
- Weibel E. R., Gil J. Electron microscopic demonstration of an extracellular duplex lining layer of alveoli. Respir Physiol. 1968 Jan;4(1):42–57. doi: 10.1016/0034-5687(68)90006-6. [DOI] [PubMed] [Google Scholar]
- Weibel E. R., Kistler G. S., Töndury G. A stereologic electron microscope study of "tubular myelin figures" in alveolar fluids of rat lungs. Z Zellforsch Mikrosk Anat. 1966;69:418–427. doi: 10.1007/BF00406293. [DOI] [PubMed] [Google Scholar]
- Williams J. V., Tierney D. F., Parker H. R. Surface forces in the lung, atelectasis, and transpulmonary pressure. J Appl Physiol. 1966 May;21(3):819–827. doi: 10.1152/jappl.1966.21.3.819. [DOI] [PubMed] [Google Scholar]