Figure 1.
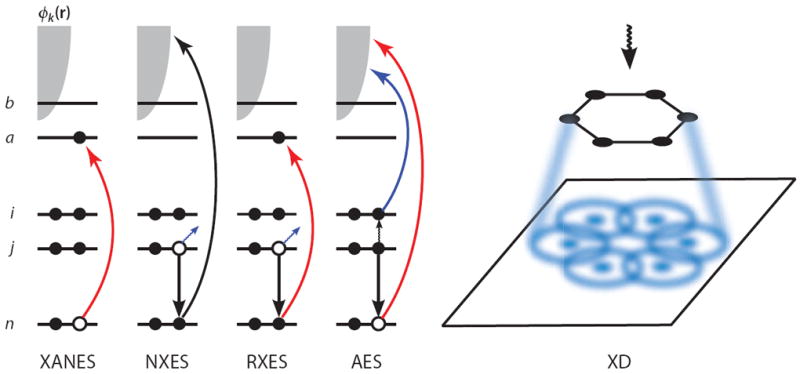
A schematic showing the electronic transitions involved in traditional, linear X-ray spectroscopy techniques. n denotes the core orbital, i and j are occupied valence orbitals, a and b are unoccupied valence orbitals, and the continuum of photoelectron states is represented by the gray shaded area. In the X-ray absorption near-edge structure (XANES), an electron is promoted from the core orbital to an unoccupied valence orbital. In nonresonant X-ray emission spectroscopy (NXES), a core electron is ejected owing to interaction with an applied field, and a valence electron falls into the core hole, emitting a red-shifted X-ray photon. In resonant X-ray emission spectroscopy (RXES), the core electron is promoted to an unoccupied valence orbital rather than being ejected, and another electron then fills the core hole while emitting a photon. In Auger electron spectroscopy (AES), the applied field ejects a core electron and a valence electron fills the hole, and the energy released by this transition is then transferred to another valence electron, which is subsequently ejected. In X-ray diffraction (XD), the elastically scattered field is spatially resolved, resulting in a diffraction pattern that can be used to elucidate structure.
