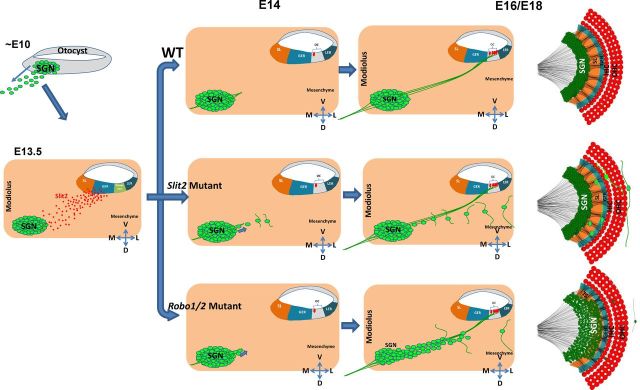
Figure 9.
A proposed model for Slit/Robo signaling in restricting SGN positioning. Early in development (∼E10), SGNs delaminate from the otocyst, migrate toward the modiolus, and settle down in the Rosenthal's canal by E13.5. Subsequently, SGNs extend their peripheral axons toward the organ of Corti (OC) at E14 and begin to form synaptic connections with HCs at E16. The SGN somas are restrained within the Rosenthal's canal during the formation of SGN-HC innervations in the wild-type cochlea. Slit2 (and possibly Slit3) secreted from the SL and GER regions acts on Robo receptors expressed in SGNs and provides the restriction force to restrain SGNs from migrating toward the cochlear epithelium. In the Slit2 mutant cochlea, a number of SGNs disperse from the Rosenthal's canal progressively and were eventually located in the space dorsal to the cochlear epithelium. During this process, their neurites progressively extend out from the soma and largely travel along the longitudinal direction and appear not to innervate HCs. In the Robo mutant, a small number of SGNs are scattered dorsal to the cochlear epithelium similarly as in the Slit2 mutant. In addition, the entire SGN territory expands progressively toward the cochlear epithelium starting from E14, resulting in a shorter SGN–HC distance as well as a broader SGN width. The more severe defect in the Robo mutant compared with the Slit2 mutant suggests that additional factors act synergistically with Slit2 on Robo receptors to restrict the SGN soma within the Rosenthal's canal, which is essential for the formation of precise patterning of SGN–HC connections.