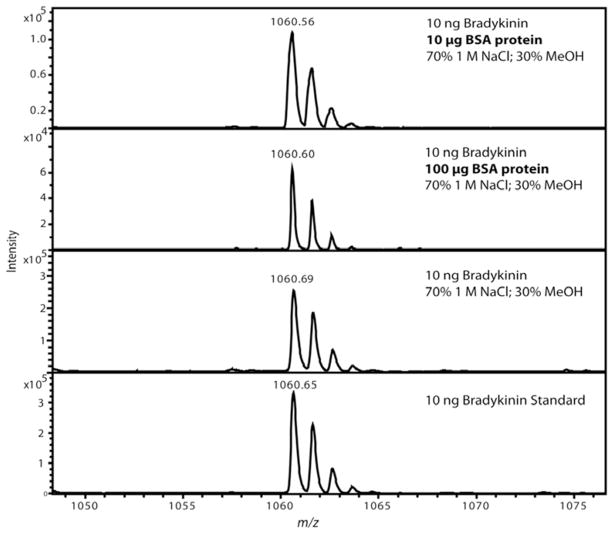
Figure 3.
Representative MALDI mass spectra after MWCO separation of a bradykinin standard showing optimized solvent conditions prevented non-specific binding to bovine serum albumin (BSA). Each experiment was performed in duplicate with representative spectrum selected for each. Two different amounts of BSA protein were tested to assess the magnitude of sample loss caused by the presence of a protein. The top panel shows 10 μg of BSA protein and the second panel shows 100 μg of BSA protein added while only 10 ng of bradykinin was added. Detectable sample loss was observed when the BSA protein was added, but panel two shows that the amount of BSA protein was 1×104 greater (equivalent to 160 fold molar excess) than bradykinin. The last two spectra were controls using a MWCO with the optimized solution in panel 3 and panel 4 using 10 ng bradykinin which was diluted to an equivalent volume as all the other experiments and directly spotted onto the MALDI plate.