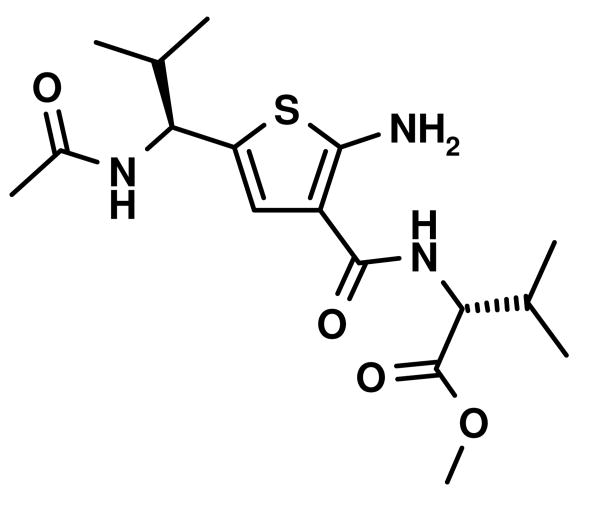
Fig. 4.
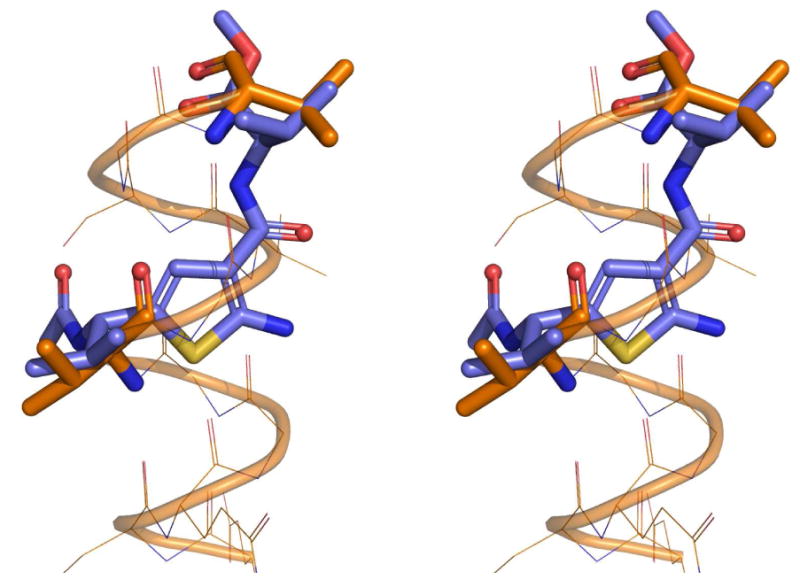
Stereoview of an overlap of an amphiphatic α-helix with two valine in i and i+6 positions (orange sticks) with a Gewald thiophene-derived dipeptide mimetic (blue sticks). The small molecule derived from aldehyde A7, cyanoacetamide B23, sulfur was deprotected and N-acylated and then energy minimized using MOLOC software.28 An important feature of the Gewald-thiophene derivatives of cyanoacetamides is the intramolecular hydrogen bonding of the 2-amino group with the 3-amide carbonyl, reducing the conformational freedom of the amide group considerably. In support of the above hypothetical structure, the intramolecular hydrogen bridge can be seen in most of the published x-ray structure analysis.4