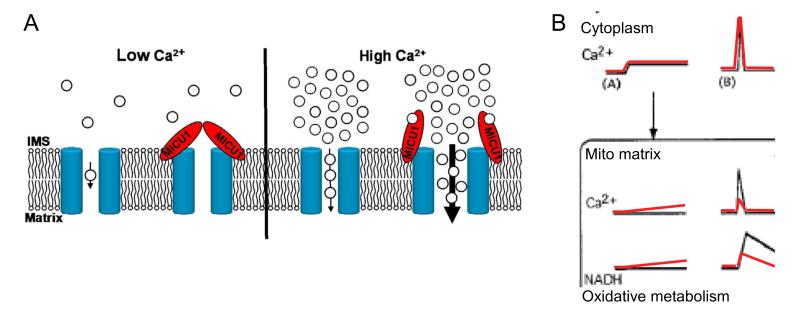
Fig6. MICU1 supports decoding of [Ca2+]c signals by stabilizing the closed state and supporting Ca2+-dependent activation of mtCU.
(A) Graphical representation of the modulation of mtCU by MICU1. (B) The model depicts the two major flaws in mitochondrial [Ca2+]c signal detection and the coupled Ca2+ control of oxidative metabolism in MICU1 deficient cells. Small [Ca2+]c elevations (A) are tuned out by Ctrl (black) mitochondria owing to MICU1-dependent thresholding; but are able to propagate to the MICU1-deficient (red) mitochondria causing stimulation and long-term exhaustion of oxidative metabolism. Large [Ca2+]c transients (B) such as the rapid short-lasting IP3R-derived high [Ca2+]c microdomains are effectively transferred to the mitochondrial matrix due to MICU1-dependent cooperative activation of mtCU. In the absence of MICU1 the efficacy of signal propagation decreases. Please note that oxidative metabolism was assessed by NAD(P)H imaging in (Hajnoczky et al., 1995) and by cellular JO2 measurement in the present study.