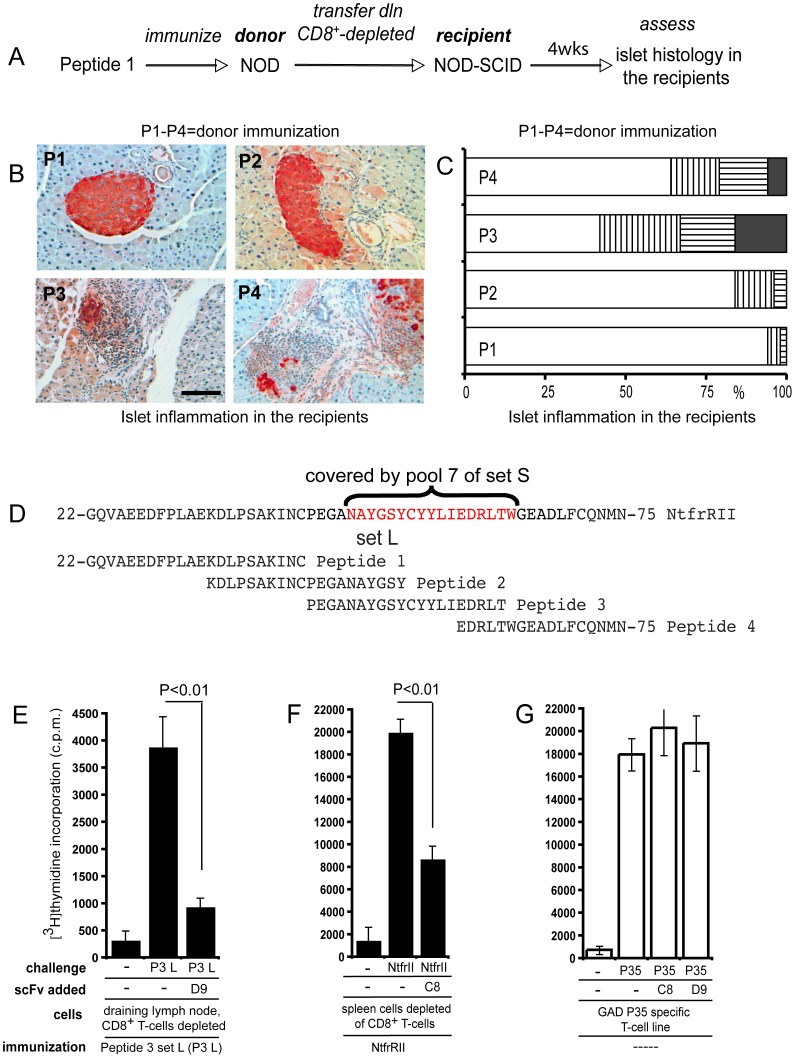
Figure 3. Autoreactive CD4+ T-cells and BscFv D9 recognize the same I-Ag7 NtfrRII peptide complex.
Four groups of NOD mice were immunized s.c. with the four peptides of set L in alum, one peptide for each group of mice. Set L covers NtfrII and by applying the procedure shown for peptide 1 (A) to the remaining peptides in set L the ability of each peptide to activate NtfrRII specific CD4+ islet self-reactive T-cells could be assessed. Set L Peptide 1 (P1) 22-GQVAEEDFPLAEKDLPSAKINC-43 Peptide 2 (P2) 34-KDLPSAKINCPEGANAYGSY-53 Peptide 3 (P3) 44-PEGANAYGSYCYYLIEDRLT-63 Peptide 4 (P4) 59-EDRLTWGEADLFCQNMN-75 This was accomplished by transferring CD4+ T-cells from the vaccinated NOD donor mice to NOD-SCID recipients and then - four weeks after transfer - studying the islet histology in the NOD-SCID recipients. Few infiltrated islets existed in recipients of CD4+ T-cells derived from P1- and P2-vaccinated donors. In contrast, as shown in B, extensive infiltration with islet destruction was observed in recipients of CD4+ T-cells from P3- and P4-vaccinated donors (scale bar: 50 µm). For each peptide a total of 60–80 islets from pancreata of three recipients were assessed for islet inflammation (C) (white bars: no infiltration; vertically striped bars: peri islet infiltration; horizontally striped bars: intra islet infiltration; black bars: islet destruction). Figure 3D demonstrates that the region of NtfrRII covered by peptide pool 7 of set S (RegII 48–64, as indicated in red), which was preferentially bound by BscFv D9 when presented in the context of I-Ag7, overlaps to a large extent with peptide 3 of set L (RegII 44–63). Since peptide 3 specific T-cells infiltrate and destroy islets it is highly likely that RegII specific autoaggressive CD4+ T-cells exist in the NOD mouse that recognize the same I-Ag7 RegII peptide complex as does BscFv D9. To test this possibility further we examined if recombinant D9 could attenuate the proliferation of CD4+ T-cells from peptide 3 set L-vaccinated NOD mice. For this purpose recombinant clone D9 (10 µg/ml) was added to a proliferation assay of spleen cells depleted of CD8+ T-cells from peptide 3 set L-vaccinated NOD mice (E). A second BscFv clone, termed C8 that suppressed proliferation of NtfrRII specific CD4+ T-cells was tested with a T-cell assay similar to that used to test D9. However, in this case donors were vaccinated with the full-length NtfrRII given that the epitope recognized by C8 in the context of I-Ag7 had not been mapped (F). To determine whether the attenuation of proliferation was antigen specific we used a previously established CD4+ T-cell line, which recognizes peptide 35 of the autoantigen glutamic acid decarboxylase (GAD) in the context of I-Ag7. GAD peptide 35 (524-SRLSKVAPVIKARMMEYGTT-543) is one of several epitopes identified in this protein that can activate NOD self-reactive CD4+ T-cells. GAD peptide 35 presented in the context of I-Ag7 should not be recognized by either C8 or D9, which are idiotypes binding to RegII (NtfrRII)-derived epitopes presented in the context of I-Ag7. Neither C8 nor D9 attenuated proliferation of the GAD peptide 35 specific T-cell line (G). Statistical evaluation was performed by t-test. Representative graphs are shown in E, F and G; three independent experiments were performed. For T-cell proliferation assays in this report triplicates were counted.