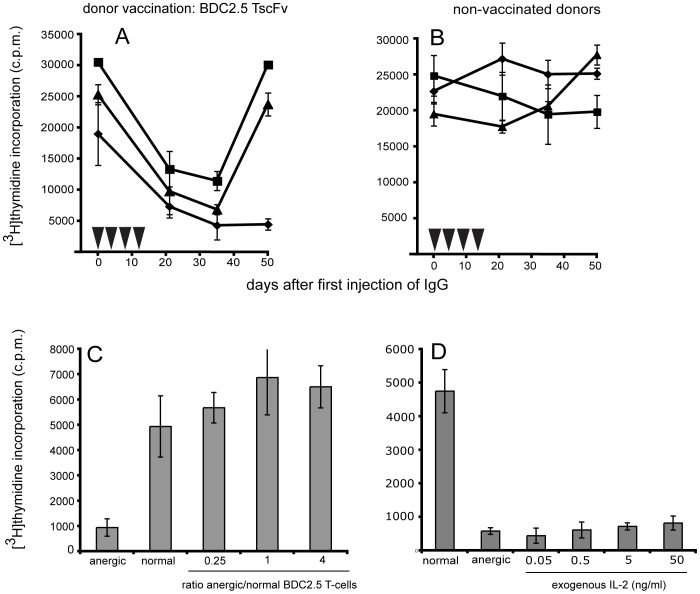
Figure 8. Effects in the BDC2.5 TCR transgenic NOD mouse (IgG).
To confirm that immunoglobulin produced by B-cells from scFv-vaccinated mice induced the anergic phase seen after B-cell transfer, IgG from BDC2.5 TscFv-vaccinated donors (A) or IgG from non-vaccinated donors (B) was transferred to BDC2.5 TCR tg recipients. Both IgGs were purified by immobilized protein A/G and administered in 4 injections to BDC2.5 TCR tg recipients (▾, 3xi.v.; 1xi.p. 5 mg/kg each injection). To functionally characterize anergic T-cells we tested whether they were able to mediate ‘bystander’ suppression ( = attenuation of normal, non-anergic T-cells) and whether exogenous IL-2 restored their responsiveness. Anergic BDC2.5 T-cells generated in vivo were isolated from recipient’s PBMCs 4–5 weeks after the first transfer of anti BDC2.5 TscFv IgG. Before co-incubation with T-cells from untreated BDC2.5 TCR tg mice at the indicated ratios, anergic T-cells were irradiated (3000rads) (C). Anergic T-cells were incubated with IL-2 at the indicated concentration (D). The mimotope concentration for both experiments was 5 µg/ml (proliferative background response for both normal and anergic BDC2.5 TCR tg was <200 c.p.m). Anergic BDC2.5 T-cells did not suppress proliferation of normal, non-anergic BDC2.5 TCR tg T-cells and did not regain their proliferative response to mimotope stimulation upon addition of exogenous IL-2. Experiments C and D are representative graphs. Two independent experiments each for (C) and (D) were performed.