| Activity assay: | A sample is collected from the tissue of interest, such as red blood cells. Xanthine–xanthine oxidase is added to the sample to generate superoxide anions (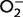 ), and then a chromagen is used as an indicator of ), and then a chromagen is used as an indicator of 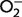 production. In the presence of SOD, production. In the presence of SOD,  concentrations are reduced, resulting in decreased colorimetric signal. However, all three SOD isoforms contribute to the activity measured, and so SOD1 activity is obtained indirectly by subtracting SOD2 and SOD3 activity from total SOD activity. This is achieved by running a parallel assay with the addition of potassium cyanide which preferentially inhibits SOD1 (Roe et al., 1988). concentrations are reduced, resulting in decreased colorimetric signal. However, all three SOD isoforms contribute to the activity measured, and so SOD1 activity is obtained indirectly by subtracting SOD2 and SOD3 activity from total SOD activity. This is achieved by running a parallel assay with the addition of potassium cyanide which preferentially inhibits SOD1 (Roe et al., 1988). |
| Gel assay: | Proteins from the tissue of interest are separated by electrophoresis in a native gel which is subsequently stained using a solution of nitro blue tetrazolium and riboflavin. Riboflavin is a source of 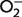 when exposed to light. The superoxide anions interact with nitro blue tetrazolium, reducing the yellow tetrazolium within the gel to a blue precipitate. This reduction reaction stains the gel blue; however, SOD inhibits this reaction, resulting in colourless bands where SOD is present. As the intensity of these bands is relative to the amount of SOD present, quantification can be inferred by measuring the intensity of the bands at the correct molecular weight using digital software (Weydert and Cullen, 2010). when exposed to light. The superoxide anions interact with nitro blue tetrazolium, reducing the yellow tetrazolium within the gel to a blue precipitate. This reduction reaction stains the gel blue; however, SOD inhibits this reaction, resulting in colourless bands where SOD is present. As the intensity of these bands is relative to the amount of SOD present, quantification can be inferred by measuring the intensity of the bands at the correct molecular weight using digital software (Weydert and Cullen, 2010). |

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.