Abstract
Background and Purpose
Sudden cardiac death is one of the leading causes of death in patients with myotonic dystrophy type 1 (DM1). It has been proposed that a prolonged QT interval is associated with sudden cardiac death in several neurological diseases, including multiple system atrophy, idiopathic Parkinson's disease, and diabetic autonomic neuropathy. However, analyses of the corrected QT (QTc) interval in DM1 patients are rare in the literature. The purposes of this study were to determine the association between the QT interval and DM1, and the affecting factors.
Methods
Thirty-nine patients diagnosed with DM1 through genetic testing were enrolled. The QTc interval (calculated using Bazett's formula: QTc=QT/√RR) was compared between these patients and 39 normal healthy controls. The clinical and laboratory factors affecting QTc interval in the patient group were investigated.
Results
The QTc interval was significantly longer in the DM1 group (411.2±44.7 msec, mean±SD) than in the normal control group (355.6±20.6 msec). Intragroup analysis revealed that a prolonged QTc interval in DM1 patients was associated with being female and older, having a longer disease duration, and exhibiting abnormal electrocardiography findings.
Conclusions
The higher incidence of sudden cardiac death in the DM1 population is associated with the observed prolonged QTc interval in those patients.
Keywords: myotonic dystrophy, corrected QT interval, Bazett's formula, sudden cardiac death
Introduction
Myotonic dystrophy type 1 (DM1) is an autosomal-dominant disorder characterized by muscular weakness, myotonia, cataract, alopecia, diabetes, thyroid dysfunction, cognitive impairment, and cardiac involvements.1-4 DM1 is caused by an unstable expansion of a cytosine-thymine-guanine (CTG) repeat in the dystrophia myotonica phosphokinase gene on chromosome 19q13.3,5-7 with the CTG repeat size being correlated positively with the disease severity and inversely with the age at onset.8-11
Sudden cardiac death, resulting mainly from conduction system defects and cardiac arrhythmias,12-15 is one of the leading causes of death in patients with DM1. The relationship between a prolonged corrected QT (QTc) interval on surface electrocardiography (EKG) and sudden cardiac death has been reported in several neurological diseases, including primary autonomic failure, diabetic autonomic neuropathy, multiple system atrophy, and idiopathic Parkinson's disease.16-18 However, analyses of the QTc interval in DM1 patients are rare in the literature.19-21
The purposes of this study were to determine the association between QTc interval and DM1, and the affecting factors.
Methods
Patients and controls
Fifty-two patients diagnosed with DM1 between 1999 and 2003 at the Samsung Medical Center (Seoul, Korea) were investigated retrospectively. All patients were diagnosed by clinical and electrophysiologic findings and confirmed by genetic evaluation confirming expanded CTG repeat size over 50 sequences on chromosome 19q13.3 by using genomic DNA extracted from leukocytes. The medical records of the 52 patients were reviewed. Clinical features including age, sex, age at onset, disease duration, and clinical manifestations were assessed. The presence of extramuscular manifestations including diabetes, thyroid disease, and cardiac arrhythmias were assessed by analyzing laboratory findings such as electrolytes, blood chemistry, thyroid function test, urine analyses, and EKG. Among the 52 patients, 8 for whom surface EKG was not conducted were excluded from the study. Five other patients were excluded because their medical records were not sufficient to assess their clinical characteristics. Therefore, 39 DM1 patients (age, 40.9±15.2 years, mean±SD; 18 males, 21 females) were enrolled. Thirty-nine normal controls (age, 41.4±13.5 years; 18 males, 21 females) were also selected to compare QTc interval with DM1 patients. The normal controls were recruited from persons who take regular check-ups; they had no history of cardiovascular diseases, neurologic disorders, endocrine disorders including diabetes mellitus and thyroid diseases, drug consumption within the previous 7 days, or psychiatric disorders. Written informed consent to participate was obtained from the controls.
Neurologic assessment
The severity of muscular weakness was scored using a muscular disability rating scale (MDRS)22 as follows: grade 1, no clinical muscular weakness (diagnosis made by electromyography, slit-lamp examination, or DNA analysis); grade 2, minimal signs (myotonia, jaw and temporal wasting, facial weakness, sternomastoids wasting/weakness, ptosis, or nasal speech, with no distal weakness except isolated digits flexor weakness); grade 3, distal weakness (no proximal weakness except isolated triceps brachii weakness); grade 4, mild or moderate proximal weakness; and grade 5, severe proximal weakness (requiring a wheelchair even for moving short distances).
QTc interval
The QT interval was measured from the onset of the QRS complex to the end of the T wave. In the presence of U waves, the QT interval was measured to the nadir of the curve between the T and U waves. The QT interval should be corrected by the RR interval according to Bazett's formula (i.e., QTc=QT/√RR) because the QT interval is always affected by the heart rate.23 The normal range of QTc is under 440 msec in males and under 450 msec in females. The QT and RR intervals were measured automatically using a Page Writer EKG recorder with an automated analyzer (HP, Palo Alto, CA, USA). The waveform analyzer was programmed to sample all QRS complexes. The QTc interval was also automatically calculated according to Bazett's formula using the established program in the EKG machine. If the EKG was checked more than twice, the obtained QTc intervals were averaged.
Data analysis
The intergroup differences in the QTc interval between the DM1 and control groups were compared using the independent t-test. The intragroup differences in the QTc interval relative to the sex, diabetes mellitus, abnormal EKG finding, MDRS score, age, age at onset, disease duration, and CTG repeat number in the DM1 group were also analyzed using the independent t-test, Kruskal-Wallis test, and univariate regression analysis. Multivariate regression analysis was conducted to assess the clinical factors affecting the QTc interval in DM1. A probability level of p<0.05 was considered to indicate a statistically significant difference.
Results
The data concerning age, sex, age at onset, disease duration, associated diabetes mellitus, abnormal EKG findings, CTG repeat numbers, and QTc interval of the DM1 patients are summarized in Table 1. Seventeen patients (43.6%) exhibited abnormal EKG findings, comprising sinus bradycardia in seven patients, first-degree atrioventricular (AV) block in five patients, left-ventricular hypertrophy in four patients, right bundle branch block in two patients, atrial fibrillation in one patient, and complete AV block in one patient. The distribution of the MDRS score, which reflects the severity of muscular weakness in DM1, was MDRS 1 in 6 patients (15.4%), MDRS 2 in 5 patients (12.8%), MDRS 3 in 15 patients (38.5%), MDRS 4 in 11 patients (28.2%), and MDRS 5 in 2 patients (5.1%).
Table 1.
General characteristics and QTc intervals in DM1 and controls (independent t-test)
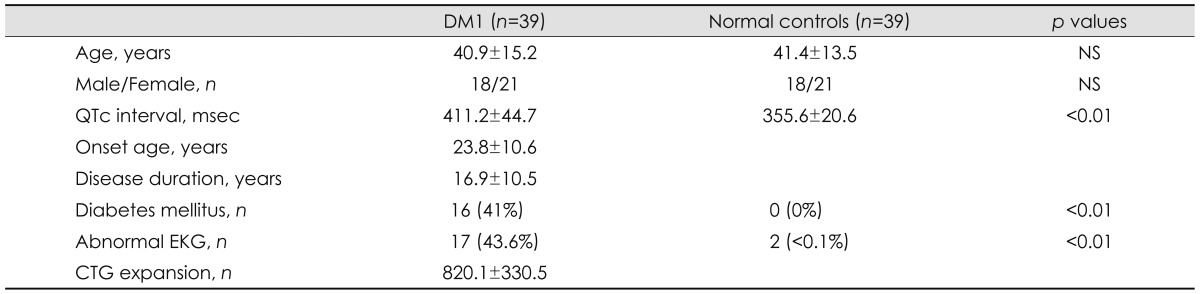
Data are expressed mean±standard deviation.
CTG: cytosine-thymine-guanine, DM1: myotonic dystrophy type 1, QTc interval: corrected QT interval.
The QTc interval was significantly longer in the DM1 group of patients (411.2±44.7 msec) than in the normal control group (355.6±20.6 msec; p<0.01). The age and sex ratio did not differ significantly between the patients (40.9±15.2 years; 18 males, 21 females) and the normal control group (41.4±13.5 years; 18 males, 21 females) (p=0.82). None of the normal controls had a history of diabetes mellitus. Two among the 39 normal controls exhibited abnormal findings (left-ventricular hypertrophy in 1 and right bundle branch block in 1).
Ten of the 39 DM1 patients (8 females and 2 males) had an abnormally prolonged QTc interval (i.e., >440 ms in males and >450 ms in females). Seven patients had diabetes mellitus and all patients had abnormal EKG findings (sinus bradycardia in 3, left-ventricular hypertrophy in 3, first-degree AV block in 2, atrial fibrillation in 1, and complete AV block in 1). None of the normal controls exhibited an abnormal prolonged QTc interval.
The associations between the QTc interval with the clinical factors of age, sex, age at onset, disease duration, associated diabetes mellitus, abnormal EKG findings, MDRS, and CTG repeat number in patients with DM1 are summarized in Table 2 and Figs. 1 and 2. The QTc interval was significantly longer in female DM1 patients with abnormal EKG findings than in their male counterparts. It also appeared to be longer in diabetic DM1 patients than in nondiabetic patients; however, the difference was not statistically significant. The QTc interval was positively correlated with DM1 patients who were older (p<0.05) and had a longer disease duration (p<0.05). The age at onset, MDRS, and CTG repeat number were not significantly associated with the QTc interval. Multivariate regression analysis revealed that only DM1 patients with abnormal EKG findings were associated with a prolonged QTc interval (Table 3).
Table 2.
The comparison the QTc interval in DM1 according to sex and associated diabetes mellitus and abnormal EKG findings (independent t-test)
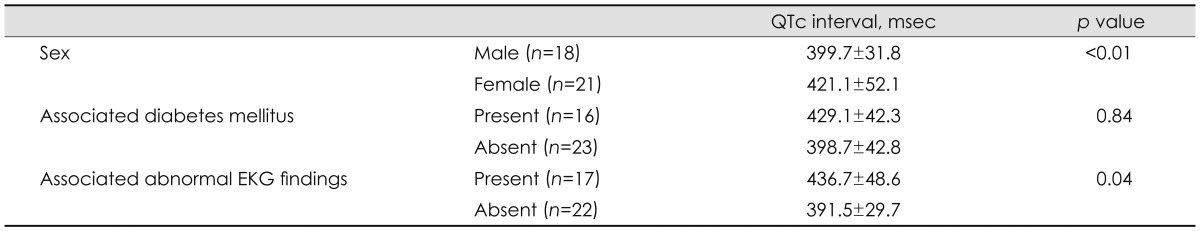
Data are expressed as mean±standard deviation.
DM1: myotonic dystrophy type 1, QTc interval: corrected QT interval.
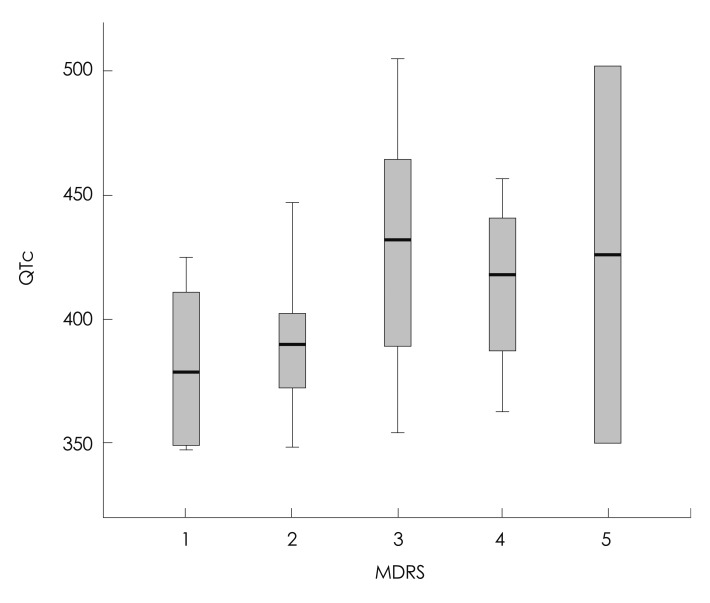
Fig. 1.
Corrected QT (QTc) interval in myotonic dystrophy type 1 according to the muscular disability rating scale (MDRS) score. In the box plots, the central line represents the median value (Kruskal-Wallis test, p=0.245), each box spans from the 25th to 75th percentiles, and error bars extend from the 10th to 90th percentiles.
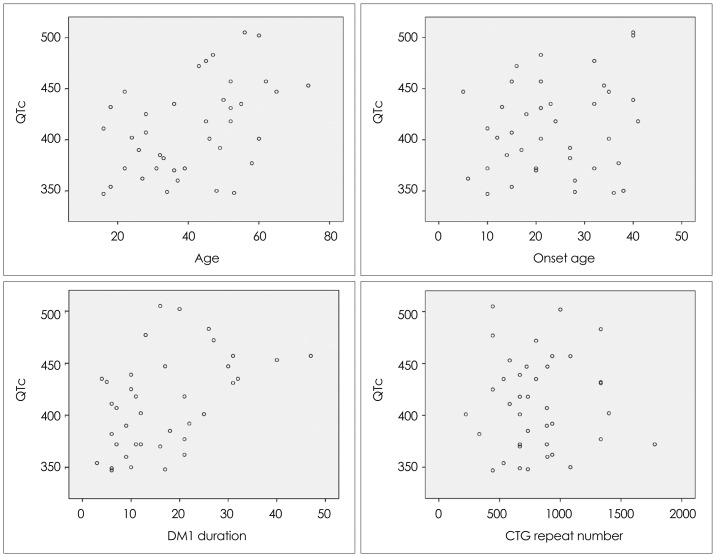
Fig. 2.
QTc interval in DM1 patients according to their age, age at onset, disease duration, and cytosine-thymine-guanine (CTG) repeat number. Univariate regression analysis for age (p<0.01, R2=0.20), age at onset (p=0.25, R2=0.04), disease duration (p<0.01, R2=0.21), and CTG repeat number (p=0.99, R2=0.00). DM1: myotonic dystrophy type 1, QTc interval: corrected QT interval.
Table 3.
The QTc interval in DM1 according to age, sex, onset age, disease duration, associated diabetes mellitus and abnormal EKG findings, MDRS, and CTG repeat number (multivariate regression analysis)
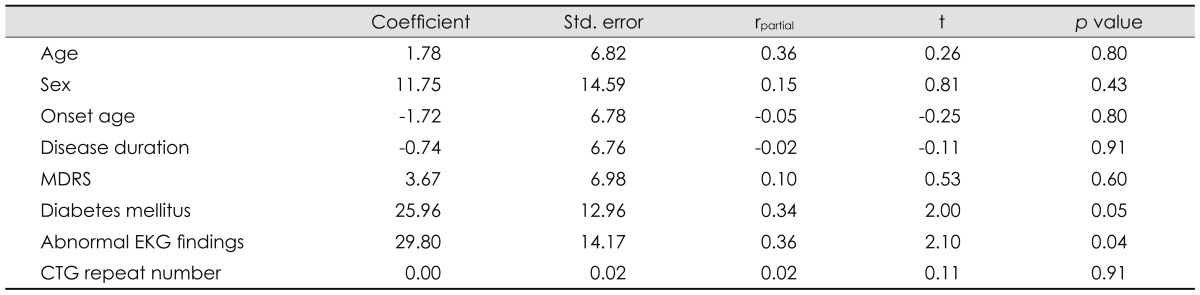
CTG: cytosine-thymine-guanine, DM1: myotonic dystrophy type 1, MDRS: muscular disability rating scale, QTc interval: corrected QT interval.
Discussion
Cardiac involvement is a frequent and significant manifestation in DM1. Conduction system defects and arrhythmias are the major abnormalities. Myocardial dysfunction, ischemic heart disease, and mitral valve prolapse are observed less frequently.10,11,14,15,24,25
Sudden cardiac death is also observed in several diseases that are accompanied by a prolonged QTc interval on surface EKG, such as long-QT syndrome, diabetic autonomic neuropathy, and multiple system atrophy. These diseases result in sudden cardiac death through their influence on the cardiovascular autonomic nervous system (ANS).16-18,26 Symptoms suggesting ANS impairment, such as respiratory impairment, syncope, dysphagia, megacolon, and voiding difficulty have been observed in patients with DM1. Involvement of the cardiac ANS in DM1 patients has been found in several investigations, although the results have been contradictory.27-30
QTc as calculated using Bazett's formula is a safe and simple method of evaluating the cardiovascular ANS. The QT interval reflects the duration of activation and recovery of the ventricular myocardium, and is influenced by cardiovascular ANS activity. Prolongation of the recovery from electrical excitation increases the likelihood of dispersing refractory consequences. Increased transmural dispersion of repolarization (i.e., the interval from Tpeak to Tend) is associated with prolonged QTc interval and transmural reentry. Therefore, QT prolongation can lead to polymorphic ventricular tachycardia or torsade de pointes, which itself may lead to ventricular fibrillation and sudden cardiac arrest. Torsade de pointes is generally thought to be induced by reactivation of calcium channels, reactivation of a delayed sodium current, or a decreased outward potassium current that results in early afterdepolarization.18,26,31,32
Since prolongation of QTc is associated with a lowered threshold of ventricular fibrillation and ventricular arrhythmia, a prolonged QT interval is associated with increased mortality in both patients with heart disease and even healthy populations.26,31-33
In a recent study with 62 DM1 patients, the QT variability index was found to be increased and the heart rate variability decreased in DM1 patients, reflecting an increased myocardial repolarization lability and increased arrhythmic risk.21
In the present study, the QTc interval was more significantly prolonged in the DM1 patient group than in the normal controls. This suggests that the higher risk of sudden cardiac death in the DM1 population is associated with that QTc prolongation. Female DM1 patients who are older with a longer disease duration and abnormal EKG findings were significantly associated with prolonged QTc interval in the intragroup analysis. The prolonged QTc interval in those who are older and have a longer disease duration may be due to profound degeneration of the myocardium and cardiac ANS. Indeed, interstitial fibrosis, fatty infiltration, and myocardial hypertrophy have been observed in endomyocardial biopsy and postmortem studies of DM1 patients.34,35 The associated abnormal EKG findings in DM1 patients reflect myocardial involvement, which may affect the prolongation of QTc. In fact, QTc prolongation was observed only in those patients with abnormal EKG findings in the multivariate regression correlation analysis.
Several potential limitations of this study should be considered. First, a prolonged QTc interval is more prevalent in patients with cardiac disease than in the normal population, and hence the results of this study may reflect the influence of cardiac disease instead of DM1. It is unclear whether or not prolonged QTc interval in DM1 is the effect of cardiac involvement associated with DM1. Cardiac involvement in DM1 is not uncommon, and hence it is necessary to determine whether the abnormal EKG findings in DM1 are the result of DM1 or other causes. Second, surface EKG does not reflect overall cardiac and cardiovascular autonomic functions. Echocardiography, 24-hour Holter monitoring, and cardiovascular autonomic function tests must be included in future studies to determine the association between a prolonged QTc interval and cardiac or cardiovascular autonomic functions in DM1. Third, although abnormal EKG findings were significant in independent t-test and multivariate analyses, the sample was small and the p value was only borderline significant (p=0.044). Fourth, the abnormal EKG findings in DM1 were nonspecific and heterogeneous. Abnormal EKG findings are not necessarily indicative of abnormal heart function and cardiac disease.
The prolonged QTc interval did not appear to be clinically significant in the female patient group, but this may have been due to the small sample.
Other limitations of this study were its retrospective design and the small sample. The QTc interval is prone to the effects of several drugs, cardiac diseases, endocrine dysfunction, electrolytes, and physical and psychological stress. However, we were unable to correct for these factors in the analysis of the QTc interval of DM1 patients. Prospective studies with larger samples should be performed to determine the association between the apparently higher incidence of sudden cardiac death and the prolonged QTc interval in DM1 patients.
The advantages of the QTc interval for assessing the cardiovascular ANS are that it is safe, simple, cost-effective, and reproducible. Therefore, the QTc interval can form the basis of a good screening test for assessing the cardiovascular ANS in DM1 patients. In particular, DM1 patients exhibiting QTc prolongation should be recommended to undergo regular and frequent EKG screening and other cardiac evaluations in order to prevent sudden cardiac death.
Acknowledgements
This work was supported by the 2012 Inje University research grant.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Gharehbaghi-Schnell EB, Finsterer J, Korschineck I, Mamoli B, Binder BR. Genotype-phenotype correlation in myotonic dystrophy. Clin Genet. 1998;53:20–26. doi: 10.1034/j.1399-0004.1998.531530105.x. [DOI] [PubMed] [Google Scholar]
- 2.Pizzuti A, Friedman DL, Caskey CT. The myotonic dystrophy gene. Arch Neurol. 1993;50:1173–1179. doi: 10.1001/archneur.1993.00540110053005. [DOI] [PubMed] [Google Scholar]
- 3.Jaspert A, Fahsold R, Grehl H, Claus D. Myotonic dystrophy: correlation of clinical symptoms with the size of the CTG trinucleotide repeat. J Neurol. 1995;242:99–104. doi: 10.1007/BF00887824. [DOI] [PubMed] [Google Scholar]
- 4.Mondelli M, Rossi A, Malandrini A, Della Porta P, Guzaai GC. Axonal motor and sensory neuropathy in myotonic dystrophy. Acta Neurol Scand. 1993;88:141–148. doi: 10.1111/j.1600-0404.1993.tb04206.x. [DOI] [PubMed] [Google Scholar]
- 5.Meola G. Clinical and genetic heterogeneity in myotonic dystrophies. Muscle Nerve. 2000;23:1789–1799. doi: 10.1002/1097-4598(200012)23:12<1789::aid-mus2>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 6.Harley HG, Brook JD, Rundle SA, Crow S, Reardon W, Buckler AJ, et al. Expansion of an unstable DNA region and phenotypic variation in myotonic dystrophy. Nature. 1992;355:545–546. doi: 10.1038/355545a0. [DOI] [PubMed] [Google Scholar]
- 7.Aslanidis C, Jansen G, Amemiya C, Shutler G, Mahadevan M, Tsilfidis C, et al. Cloning of the essential myotonic dystrophy region and mapping of the putative defect. Nature. 1992;355:548–551. doi: 10.1038/355548a0. [DOI] [PubMed] [Google Scholar]
- 8.Harley HG, Rundle SA, MacMillan JC, Myring J, Brook JD, Crow S, et al. Size of the unstable CTG repeat sequence in relation to phenotype and parental transmission in myotonic dystrophy. Am J Hum Genet. 1993;52:1164–1174. [PMC free article] [PubMed] [Google Scholar]
- 9.Ashizawa T, Dunne CJ, Dubel JR, Perryman MB, Epstein HF, Boerwinkle E, et al. Anticipation in myotonic dystrophy. I. Statistical verification based on clinical and haplotype findings. Neurology. 1992;42:1871–1877. doi: 10.1212/wnl.42.10.1871. [DOI] [PubMed] [Google Scholar]
- 10.Melacini P, Villanova C, Menegazzo E, Novelli G, Danieli G, Rizzoli G, et al. Correlation between cardiac involvement and CTG trinucleotide repeat length in myotonic dystrophy. J Am Coll Cardiol. 1995;25:239–245. doi: 10.1016/0735-1097(94)00351-p. [DOI] [PubMed] [Google Scholar]
- 11.Tokgozoglu LS, Ashizawa T, Pacifico A, Armstrong RM, Epstein HF, Zoghbi WA. Cardiac involvement in a large kindred with myotonic dystrophy. Quantitative assessment and relation to size of CTG repeat expansion. JAMA. 1995;274:813–819. [PubMed] [Google Scholar]
- 12.Finsterer J, Gharehbaghi-Schnell E, Stöllberger C, Fheodoroff K, Seiser A. Relation of cardiac abnormalities and CTG-repeat size in myotonic dystrophy. Clin Genet. 2001;59:350–355. doi: 10.1034/j.1399-0004.2001.590509.x. [DOI] [PubMed] [Google Scholar]
- 13.Sabovic M, Medica I, Logar N, Mandić E, Zidar J, Peterlin B. Relation of CTG expansion and clinical variables to electrocardiogram conduction abnormalities and sudden death in patients with myotonic dystrophy. Neuromuscul Disord. 2003;13:822–826. doi: 10.1016/s0960-8966(03)00138-x. [DOI] [PubMed] [Google Scholar]
- 14.Pelargonio G, Dello Russo A, Sanna T, De Martino G, Bellocci F. Myotonic dystrophy and the heart. Heart. 2002;88:665–670. doi: 10.1136/heart.88.6.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mathieu J, Allard P, Potvin L, Prévost C, Bégin P. A 10-year study of mortality in a cohort of patients with myotonic dystrophy. Neurology. 1999;52:1658–1662. doi: 10.1212/wnl.52.8.1658. [DOI] [PubMed] [Google Scholar]
- 16.Glickstein JS, Schwartzman D, Friedman D, Rutkowski M, Axelrod FB. Abnormalities of the corrected QT interval in familial dysautonomia: an indicator of autonomic dysfunction. J Pediatr. 1993;122:925–928. doi: 10.1016/s0022-3476(09)90022-1. [DOI] [PubMed] [Google Scholar]
- 17.Lo SS, Mathias CJ, Sutton MS. QT interval and dispersion in primary autonomic failure. Heart. 1996;75:498–501. doi: 10.1136/hrt.75.5.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deguchi K, Sasaki I, Tsukaguchi M, Kamoda M, Touge T, Takeuchi H, et al. Abnormalities of rate-corrected QT intervals in Parkinson's disease-a comparison with multiple system atrophy and progressive supranuclear palsy. J Neurol Sci. 2002;199:31–37. doi: 10.1016/s0022-510x(02)00079-5. [DOI] [PubMed] [Google Scholar]
- 19.Breton R, Mathieu J. Usefulness of clinical and electrocardiographic data for predicting adverse cardiac events in patients with myotonic dystrophy. Can J Cardiol. 2009;25:e23–e27. doi: 10.1016/s0828-282x(09)70479-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Groh WJ, Groh MR, Saha C, Kincaid JC, Simmons Z, Ciafaloni E, et al. Electrocardiographic abnormalities and sudden death in myotonic dystrophy type 1. N Engl J Med. 2008;358:2688–2697. doi: 10.1056/NEJMoa062800. [DOI] [PubMed] [Google Scholar]
- 21.Magrì D, Piccirillo G, Bucci E, Pignatelli G, Cauti FM, Morino S, et al. Increased temporal dispersion of myocardial repolarization in myotonic dystrophy type 1: beyond the cardiac conduction system. Int J Cardiol. 2012;156:259–264. doi: 10.1016/j.ijcard.2010.10.132. [DOI] [PubMed] [Google Scholar]
- 22.Mathieu J, De Braekeleer M, Prévost C, Boily C. Myotonic dystrophy: clinical assessment of muscular disability in an isolated population with presumed homogeneous mutation. Neurology. 1992;42:203–208. doi: 10.1212/wnl.42.1.203. [DOI] [PubMed] [Google Scholar]
- 23.Hannan PJ, Crow RS. Concerning the units for the QT interval corrected by Bazett's formula. Circulation. 1997;96:3799. [PubMed] [Google Scholar]
- 24.Melacini P, Buja G, Fasoli G, Angelini C, Armani M, Scognamiglio R, et al. The natural history of cardiac involvement in myotonic dystrophy: an eight-year follow-up in 17 patients. Clin Cardiol. 1988;11:231–238. doi: 10.1002/clc.4960110407. [DOI] [PubMed] [Google Scholar]
- 25.Hayashi Y, Ikeda U, Kojo T, Nishinaga M, Miyashita H, Kuroda T, et al. Cardiac abnormalities and cytosine-thymine-guanine trinucleotide repeats in myotonic dystrophy. Am Heart J. 1997;134(2 Pt 1):292–297. doi: 10.1016/s0002-8703(97)70137-6. [DOI] [PubMed] [Google Scholar]
- 26.Diedrich A, Jordan J, Shannon JR, Robertson D, Biaggioni I. Modulation of QT interval during autonomic nervous system blockade in humans. Circulation. 2002;106:2238–2243. doi: 10.1161/01.cir.0000035241.76918.6c. [DOI] [PubMed] [Google Scholar]
- 27.Di Leo R, Rodolico C, De Gregorio C, Recupero A, Coglitore S, Annesi G, et al. Cardiovascular autonomic control in myotonic dystrophy type 1: a correlative study with clinical and genetic data. Neuromuscul Disord. 2004;14:136–141. doi: 10.1016/j.nmd.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 28.Olofsson BO, Niklasson U, Forsberg H, Bjerle P, Andersson S, Henriksson A. Assessment of autonomic nerve function in myotonic dystrophy. J Auton Nerv Syst. 1990;29:187–192. doi: 10.1016/0165-1838(90)90144-8. [DOI] [PubMed] [Google Scholar]
- 29.Pierangeli G, Lugaresi A, Contin M, Martinelli P, Montagna P, Parchi P, et al. Autonomic nervous system function in myotonic dystrophy. Ital J Neurol Sci. 1992;13:589–592. doi: 10.1007/BF02233402. [DOI] [PubMed] [Google Scholar]
- 30.Inoue K, Ogata H, Matsui M, Hayano J, Miyake S, Kumashiro M, et al. Assessment of autonomic function in myotonic dystrophy by spectral analysis of heart-rate variability. J Auton Nerv Syst. 1995;55:131–134. doi: 10.1016/0165-1838(95)00040-5. [DOI] [PubMed] [Google Scholar]
- 31.Rana BS, Band MM, Ogston S, Morris AD, Pringle SD, Struthers AD. Relation of QT interval dispersion to the number of different cardiac abnormalities in diabetes mellitus. Am J Cardiol. 2002;90:483–487. doi: 10.1016/s0002-9149(02)02518-3. [DOI] [PubMed] [Google Scholar]
- 32.Behr E, Wood DA, Wright M, Syrris P, Sheppard MN, Casey A, et al. Cardiological assessment of first-degree relatives in sudden arrhythmic death syndrome. Lancet. 2003;362:1457–1459. doi: 10.1016/s0140-6736(03)14692-2. [DOI] [PubMed] [Google Scholar]
- 33.Rautaharju PM, Nelson JC, Kronmal RA, Zhang ZM, Robbins J, Gottdiener JS, et al. Usefulness of T-axis deviation as an independent risk indicator for incident cardiac events in older men and women free from coronary heart disease (the Cardiovascular Health Study) Am J Cardiol. 2001;88:118–123. doi: 10.1016/s0002-9149(01)01604-6. [DOI] [PubMed] [Google Scholar]
- 34.Phillips MF, Harper PS. Cardiac disease in myotonic dystrophy. Cardiovasc Res. 1997;33:13–22. doi: 10.1016/s0008-6363(96)00163-0. [DOI] [PubMed] [Google Scholar]
- 35.Bhakta D, Lowe MR, Groh WJ. Prevalence of structural cardiac abnormalities in patients with myotonic dystrophy type I. Am Heart J. 2004;147:224–227. doi: 10.1016/j.ahj.2003.08.008. [DOI] [PubMed] [Google Scholar]