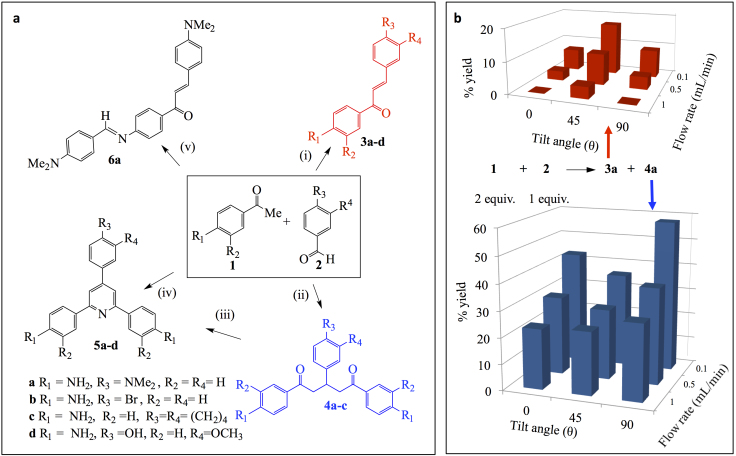
Figure 4. Synthesis of 2,4,6-triarylpyridienes.
(a) Reaction conditions: (i) 1:1 equivalent of ketone (1) and aldehyde (2), NaOH (1 eqv.), 80°C (or room temperature for 3b and 3c), PEG300, confined mode VFD, θ = 45°, 7000 rpm, 30 mins, percentage yield: 52% 3a, 65% 3b, 61% 3c, 20% 3d; (ii) 2:1 equivalent of ketone (1) and aldehyde (2), NaOH (2 eqv.), 80°C, PEG300 (or 1-propanol for 4a), continuous flow mode VFD, θ = 0°, 7000 rpm, 0.1 mL/min, percentage yield: 43% 4a, 48% 4b, 45% 4c; (iii) NH4OAc (excess), 100°C, PEG300, batch mode9; percentage yield 94% 5a, 78% 5b, 91% 5c; (iv) 2:1 equivalent of ketone (1) and aldehyde (2), NH4OAc (excess), 80°C, PEG300, continuous flow mode VFD, θ = 45°, 7000 rpm, 0.5 mL/min, percentage yield: 38% 5a, 21% 5b, 19% 5c, 45% 5d; (v) Batch mode, 90% 6a9. (b) Product distribution for a 2:1 reaction of 1 and 2, in generating a mixture of 3a and 4a.