Table 2.
Strategies for Blinding Key Players in Behavioral Trials
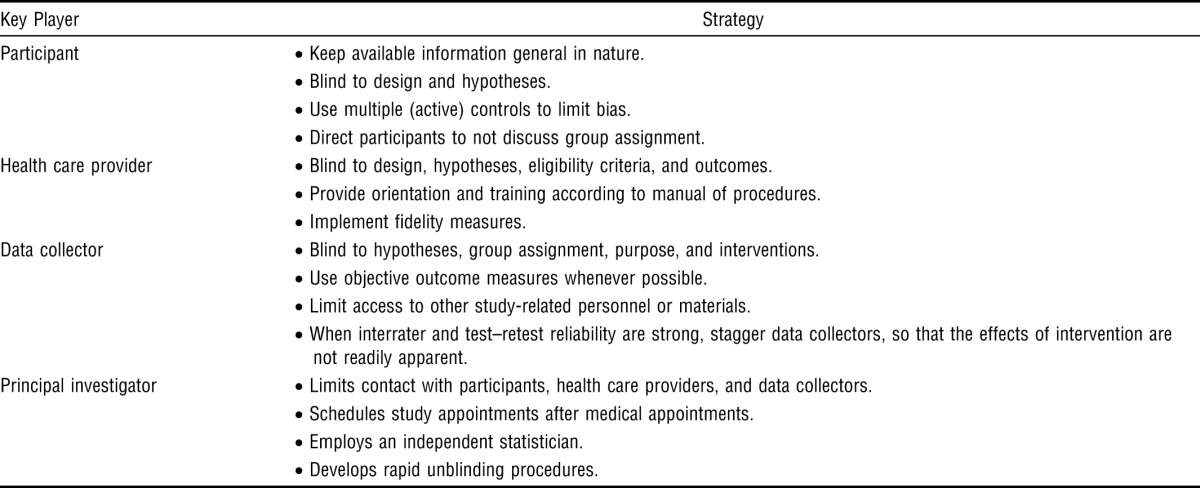
| Key Player | Strategy |
| Participant | • Keep available information general in nature. |
| • Blind to design and hypotheses. | |
| • Use multiple (active) controls to limit bias. | |
| • Direct participants to not discuss group assignment. | |
| Health care provider | • Blind to design, hypotheses, eligibility criteria, and outcomes. |
| • Provide orientation and training according to manual of procedures. | |
| • Implement fidelity measures. | |
| Data collector | • Blind to hypotheses, group assignment, purpose, and interventions. |
| • Use objective outcome measures whenever possible. | |
| • Limit access to other study-related personnel or materials. | |
| • When interrater and test–retest reliability are strong, stagger data collectors, so that the effects of intervention are not readily apparent. | |
| Principal investigator | • Limits contact with participants, health care providers, and data collectors. |
| • Schedules study appointments after medical appointments. | |
| • Employs an independent statistician. | |
| • Develops rapid unblinding procedures. |
