Abstract
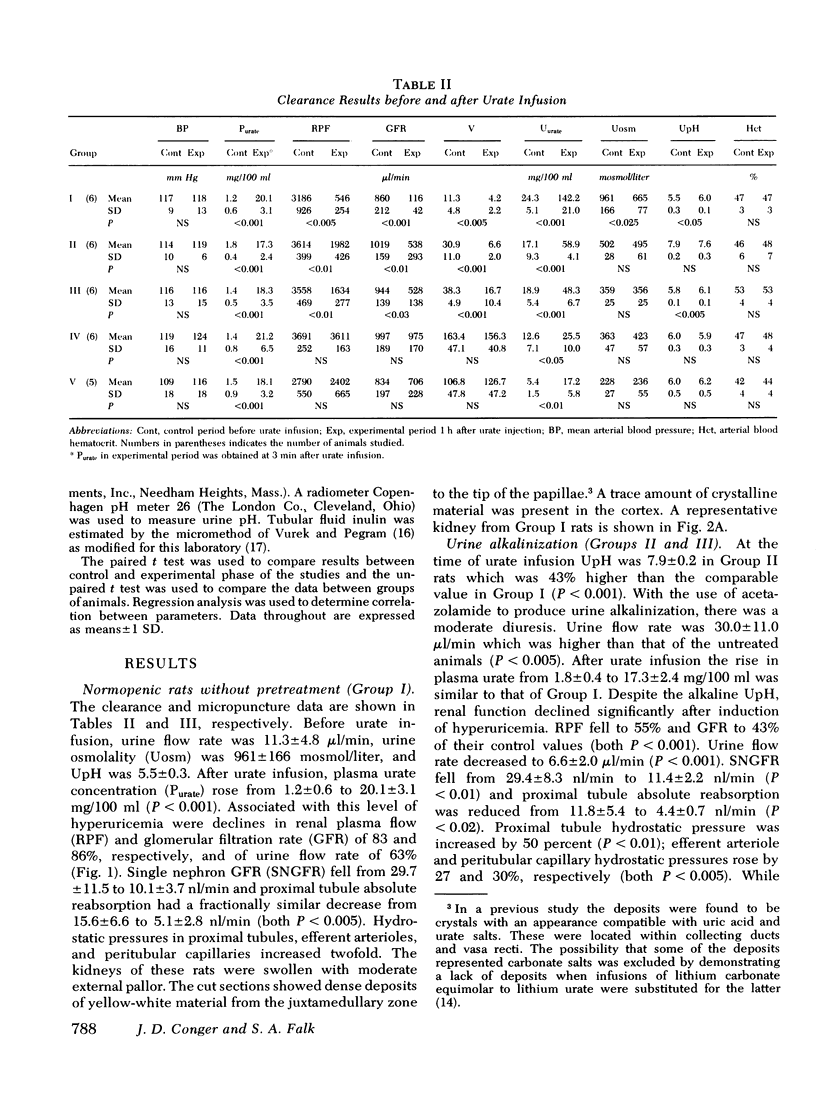
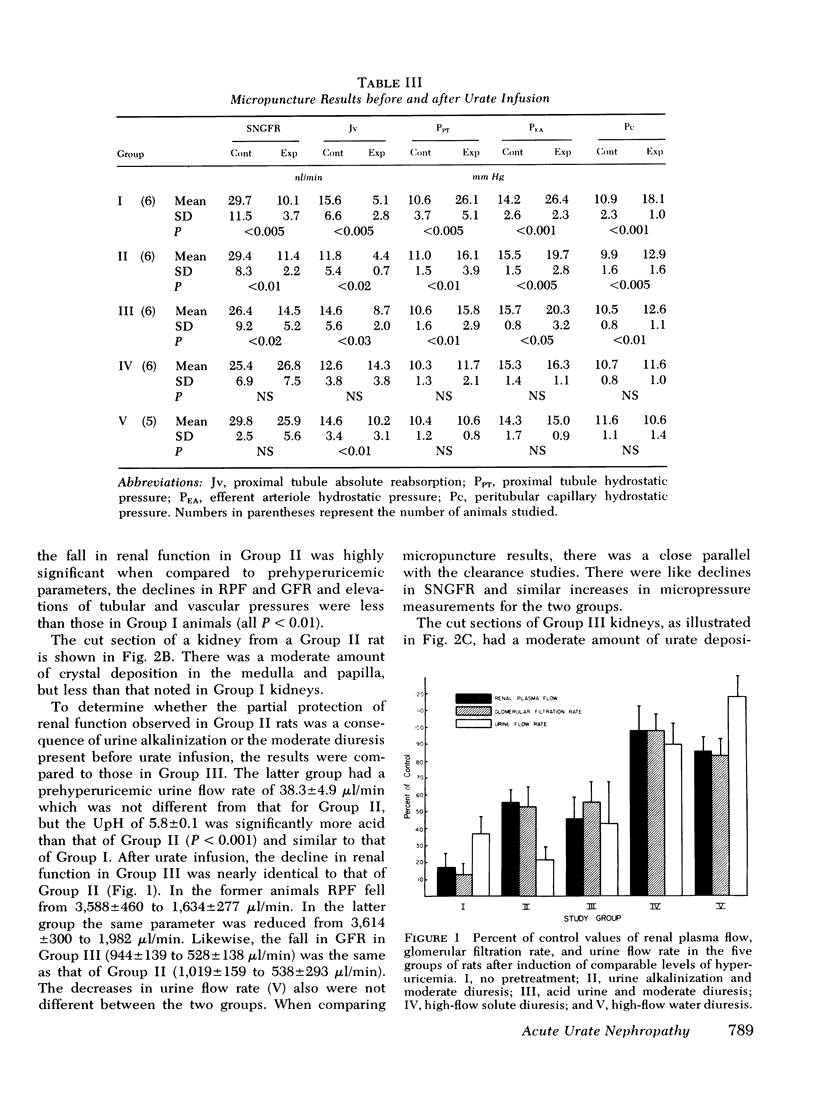
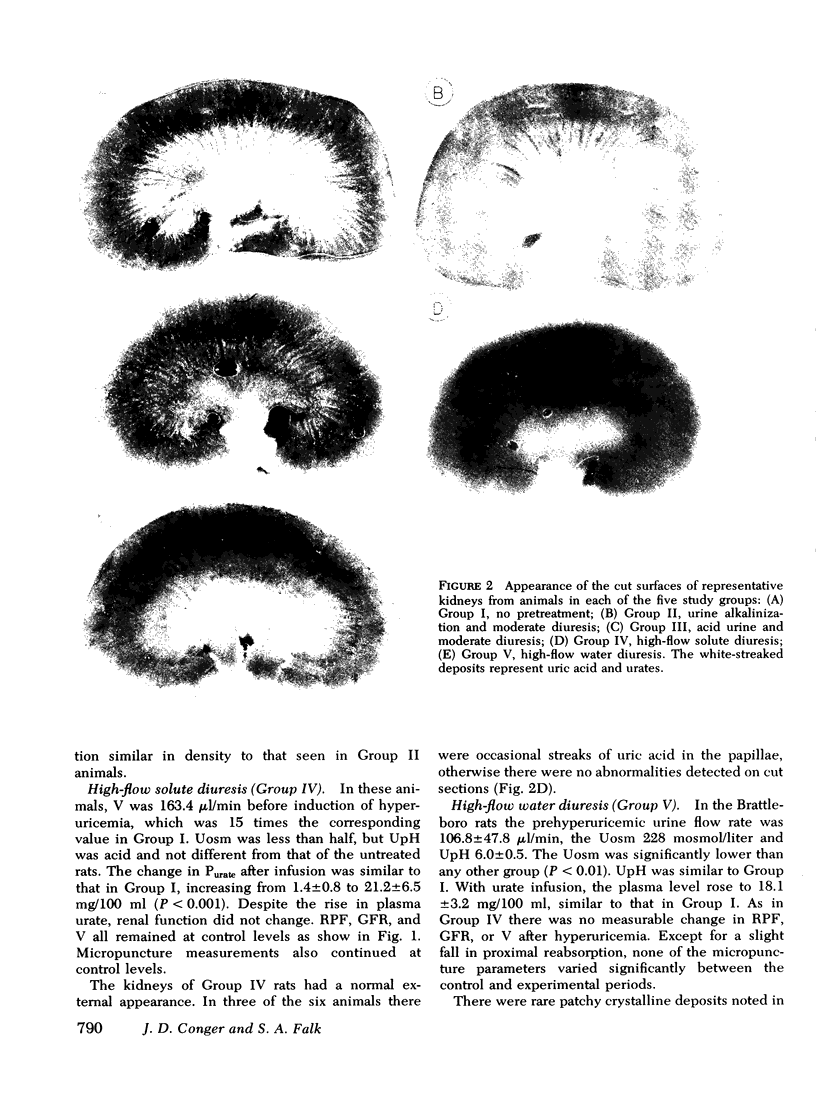
Tubular fluid flow, urine osmolality, and pH were selectively altered to determine the relative protective roles of these factors in a rat model of acute urate nephrophathy. Various prehyper uricemic conditions were established in five groups of animals: (a) normopenic Wistar rats given no pretreatment (Group I); (b) Wistar rats given acetazolamide, 20 mg/kg, and isotonic NaHCO3 to produce urine alkalinization (Group II); (c) Wistar rats in which a moderate diuresis, similar to that observed in Group II but without urine alkalinization, was induced with furosemide, 2 mg/kg (Group III); (d) Wistar rats in which a high-flow solute diuresis was induced with furosemide, 15 mg/kg (Group IV); (e) Brattleboro rats, homozygous for pituitary diabetes insipidus, that had a spontaneous high-flow water diuresis (Group V). A comparable level of hyperuricemia (19.4+/-2.2 mg/100 ml) was achieved in all animals with intravenous urate infusion. Clearance and micropuncture studies were performed before and 1 h after induction of hyperuicemia. Group I rats had mean falls in renal plasma flow and glomerular filtration rate of 83 and 86%, respectively; nephron filtration rate decreased 66%, and tubular and microvascular pressures increased twofold. In Group II there were 45 and 47% declines in renal plasma flow and glomerular filtration rate, respectively, a 66% fall in nephron filtration rate, and a 30% increase in tubular and vascular pressures. Moderate amounts of urate were seen in the kidneys. Group III had changes in renal function identical to Group II suggesting that the moderate prehyperuricemic diuresis in the latter group and not urine alkalinization produced the partial protection observed. Groups IV and V were completely and comparably protected with renal function studies unchanged from controls. It is concluded that high tubular fluid flow, whether induced by a solute or water diuresis, is the primary mechanism of protection in acute urate nephropathy. At most, urine alkalinization plays a minor preventive role.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartoli E., Conger J. D., Earley L. E. Effect of intraluminal flow on proximal tubular reabsorption. J Clin Invest. 1973 Apr;52(4):843–849. doi: 10.1172/JCI107248. [DOI] [PMC free article] [PubMed] [Google Scholar]
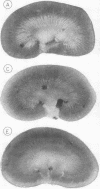
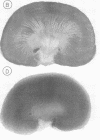
- Conger J. D., Falk S. A., Guggenheim S. J., Burke T. J. A micropuncture study of the early phase of acute urate nephropathy. J Clin Invest. 1976 Sep;58(3):681–689. doi: 10.1172/JCI108514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conger J. D., Rhoads H. N., Christie S. N., Burke T. J. A modification of the fluorescence method for micro-inulin determinations. Kidney Int. 1975 Nov;8(5):334–337. doi: 10.1038/ki.1975.121. [DOI] [PubMed] [Google Scholar]
- EPSTEIN F. H., PIGEON G. EXPERIMENTAL URATE NEROPATHY: STUDIES OF THE DISTRIBUTION OF URATE IN RENAL TISSUE. Nephron. 1964;1:144–157. doi: 10.1159/000179328. [DOI] [PubMed] [Google Scholar]
- FREI E., 3rd, BENTZEL C. J., RIESELBACH R., BLOCK J. B. RENAL COMPLICATIONS OF NEOPLASTIC DISEASE. J Chronic Dis. 1963 Jul;16:757–776. doi: 10.1016/0021-9681(63)90010-9. [DOI] [PubMed] [Google Scholar]
- Greger R., Lang F., Deetjen P. Handling of uric acid by the rat kidney. I. Microanalysis of uric acid in proximal tubular fluid. Pflugers Arch. 1971;324(4):279–287. doi: 10.1007/BF00592456. [DOI] [PubMed] [Google Scholar]
- Handa S. P. Acute renal railure in association with hyperuricemia: its recovery with ethacrynic acid. South Med J. 1971 Jun;64(6):676–678. doi: 10.1097/00007611-197106000-00008. [DOI] [PubMed] [Google Scholar]
- KRITZLER R. A. Anuria complicating the treatment of leukemia. Am J Med. 1958 Oct;25(4):532–538. doi: 10.1016/0002-9343(58)90042-1. [DOI] [PubMed] [Google Scholar]
- Kjellstrand C. M., Cambell D. C., 2nd, von Hartitzsch B., Buselmeier T. J. Hyperuricemic acute renal failure. Arch Intern Med. 1974 Mar;133(3):349–359. [PubMed] [Google Scholar]
- Klinenberg J. R., Bluestone R., Schlosstein L., Waisman J., Whitehouse M. W. Urate deposition disease. How is it regulated and how can it be modified? Ann Intern Med. 1973 Jan;78(1):99–111. doi: 10.7326/0003-4819-78-1-99. [DOI] [PubMed] [Google Scholar]
- Krakoff I. H. Use of allopurinol in preventing hyperuricemia in leukemia and lymphoma. Cancer. 1966 Nov;19(11):1489–1496. doi: 10.1002/1097-0142(196611)19:11<1489::aid-cncr2820191105>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Malnic G., De Mello Aires M., Giebisch G. Micropuncture study of renal tubular hydrogen ion transport in the rat. Am J Physiol. 1972 Jan;222(1):147–158. doi: 10.1152/ajplegacy.1972.222.1.147. [DOI] [PubMed] [Google Scholar]
- Mudge G. H. The renal tubular transport of urate. Arthritis Rheum. 1965 Oct;8(5):686–693. doi: 10.1002/art.1780080428. [DOI] [PubMed] [Google Scholar]
- RIESELBACH R. E., BENTZEL C. J., COTLOVE E., FREI E., 3rd, FREIREICH E. J. URIC ACID EXCRETION AND RENAL FUNCTION IN THE ACUTE HYPERURICEMIA OF LEUKEMIA. PATHOGENESIS AND THERAPY OF URIC ACID NEPHROPATHY. Am J Med. 1964 Dec;37:872–883. doi: 10.1016/0002-9343(64)90130-5. [DOI] [PubMed] [Google Scholar]
- Steinberg S. M., Galen M. A., Lazarus J. M., Lowrie E. G., Hampers C. L., Jaffe N. Hemodialysis for acute anuric uric acid nephropathy. Am J Dis Child. 1975 Aug;129(8):956–958. doi: 10.1001/archpedi.1975.02120450062014. [DOI] [PubMed] [Google Scholar]
- WEINTRAUB L. R., PENNER J. A., MEYERS M. C. ACUTE URIC ACID NEPHROPATHY IN LEUKEMIA. REPORT OF A CASE TREATED WITH PERITONEAL DIALYSIS. Arch Intern Med. 1964 Jan;113:111–114. doi: 10.1001/archinte.1964.00280070113018. [DOI] [PubMed] [Google Scholar]
- Watts R. W., Watkins P. J., Matthias J. Q., Gibbs D. A. Allopurinal and acute uric acid nephropathy. Br Med J. 1966 Jan 22;1(5481):205–208. doi: 10.1136/bmj.1.5481.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinman E. J., Eknoyan G., Suki W. N. The influence of the extracellular fluid volume on the tubular reabsorption of uric acid. J Clin Invest. 1975 Feb;55(2):283–291. doi: 10.1172/JCI107931. [DOI] [PMC free article] [PubMed] [Google Scholar]