Abstract
Background
LCP-Tacro is an extended-release formulation of tacrolimus designed for once-daily dosing. Phase 1 studies demonstrated greater bioavailability to twice-daily tacrolimus capsules and no new safety concerns.
Methods
In this phase 2 study, adult stable kidney transplant patients on tacrolimus capsules (Prograf) twice-daily were converted to tacrolimus tablets (LCP-Tacro) once-daily; patients continued on LCP-Tacro once-daily for days 8 to 21; trough levels were to be maintained between 5 and 15 ng/mL; 24-hr pharmacokinetic assessments were done on days 7 (baseline pre-switch), 14, and 21.
Results
Forty-seven patients completed LCP-Tacro dosing per protocol. The mean conversion ratio was 0.71. Pharmacokinetic data demonstrated consistent exposure (AUC) at the lower conversion dose. Cmax (P=0.0001), Cmax/Cmin ratio (P<0.001), percent fluctuation (P<0.0001), and swing (P=0.0004) were significantly lower and Tmax significantly (P<0.001) longer for LCP-Tacro versus Prograf. AUC24 and Cmin correlation coefficients after 7 and 14 days of therapy were 0.86 or more, demonstrating a robust correlation between LCP-Tacro tacrolimus exposure and trough levels. There were three serious adverse events; none were related to study drug and all were resolved.
Conclusions
Stable kidney transplant patients can be safely converted from Prograf twice-daily to LCP-Tacro. The greater bioavailability of LCP-Tacro allows for once-daily dosing and similar (AUC) exposure at a dose approximately 30% less than the total daily dose of Prograf. LCP-Tacro displays flatter kinetics characterized by significantly lower peak-trough fluctuations.
Keywords: Tacrolimus, Sustained-release preparations, Kidney transplantation, PK, MeltDose, LCP-Tacro, Flatter kinetics
Tacrolimus was approved by the U.S. Food and Drug Administration in April 1994 for the prophylaxis of organ rejection in patients receiving allogeneic liver transplants under the brand name Prograf (tacrolimus capsules; Astellas Pharma US, Deerfield, IL). Currently, Prograf is approved for the prophylaxis of organ rejection in patients receiving allogeneic liver, kidney, or heart transplants. Tacrolimus is one of the most widely used immunosuppression drugs in kidney transplantation (1). The Organ Procurement and Transplantation Network/Scientific Registry of Transplant Recipients 2011 Annual Data Report shows that approximately 90% of recipients transplanted in 2010 were initiated on tacrolimus as part of their immunosuppression regimen and were continuing to take tacrolimus at 1 year after transplantation (1).
Although Prograf capsules have been proven to be highly effective in preventing graft rejection (2), the drug exhibits significant interindividual and intraindividual variability of its absorption and metabolism of tacrolimus. Because of this variability, standard dosing or total daily dose is not an accurate predictor of drug exposure (3). In clinical use, tacrolimus trough blood concentrations are measured to ensure efficacy and safety (3). Furthermore, as a result of the combination of poor water solubility, presystemic metabolism of tacrolimus in the gastrointestinal tract and activity of the P-glycoprotein efflux pump found in the enterocytes of the gastrointestinal tract, Prograf exhibits relatively low bioavailability of tacrolimus (17%±10% in adult kidney transplant patients) (4).
Currently, lifelong monitoring of tacrolimus blood levels is required for transplant patients. In addition to the management of tacrolimus blood levels being complicated by variable intrapatient and interpatient absorption, Prograf requires twice-daily dosing. Multiple daily dosing is associated with increased risk for poor adherence, a complication that can lead to acute rejection, and, in serious cases, graft loss (5). Therefore, a once-daily tacrolimus dosing regimen could improve patient adherence with drug administration.
An extended-release formulation of tacrolimus designed for once-daily administration (LCP-Tacro tablets; Veloxis Pharmaceuticals, Hørsholm, Denmark) has been developed utilizing a proprietary MeltDose drug delivery technology (Veloxis Pharmaceuticals), which is designed to enhance the bioavailability of drugs with low water solubility. The MeltDose process enhances the absorption of drug substances by creation of a solid dispersion, or a solid solution, of the drug substance through a physical process coined “controlled agglomeration.” Extended-release products are designed to release their medication in a controlled manner at a predetermined rate, duration, and location to achieve and maintain optimum therapeutic blood levels of drug. Two important parameters to consider when evaluating the controlled delivery of drugs are fluctuation and swing (6, 7). Percent fluctuation is the peak-to-trough change in drug concentrations around the average concentration and the percent swing is the peak-to-trough change in drug concentrations relative to the minimum concentration. Less fluctuation and swing is desirable, we refer to this profile as flatter kinetics, in a controlled release drug. Reduced fluctuations in drug plasma concentrations may result in a more continuous effect and the avoidance of high peak concentrations may reduce the incidence and/or intensity of drug toxicity-related adverse events (AEs) (8).
The present study compared the pharmacokinetics (PK) and safety of LCP-Tacro tablets once-daily with Prograf capsules twice-daily in stable kidney transplant recipients.
RESULTS
Patient Disposition and Baseline Characteristics
There were 78 patients screened; 60 patients were enrolled from 10 centers. Of the 60 patients who were enrolled, 51 were started on LCP-Tacro and 47 completed the study. Figure 1 shows patient disposition, including reasons for dropout. More than half (68.3%) of the patients were male. The mean age was 45.6 years; most patients were either white (53.3%) or black (43.3%). The mean±SD number of years from transplantation to study enrolment was 2.62±2.21, with a range of 0.42 to 8.70 years.
FIGURE 1.
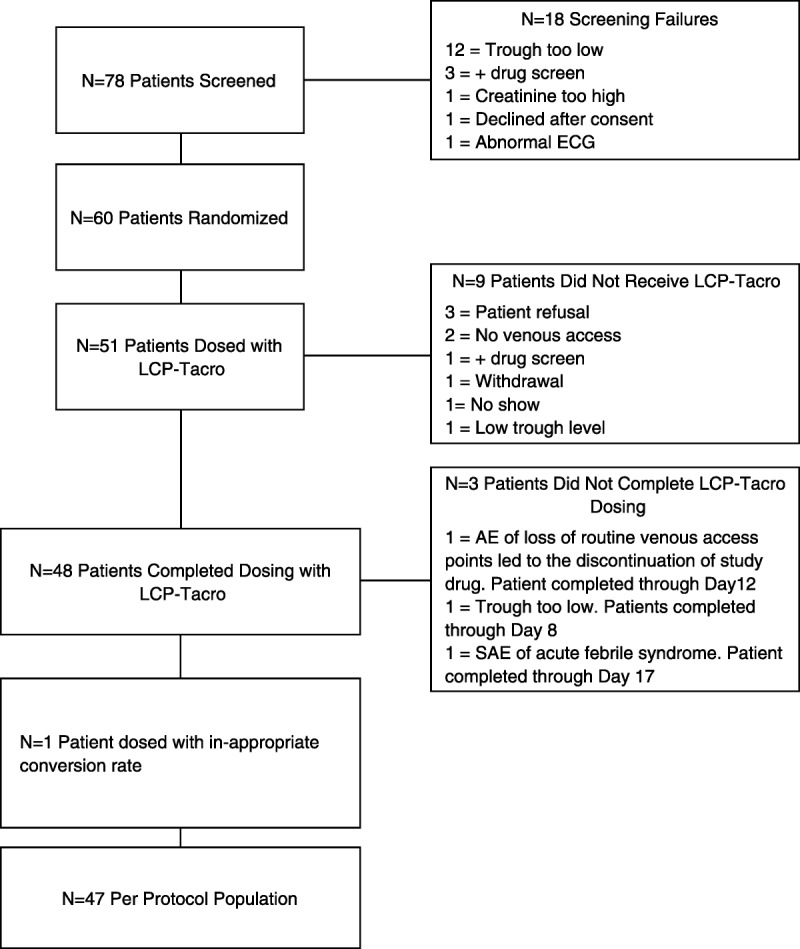
Patient disposition.
Pharmacokinetics
Results of the relative tacrolimus bioavailability analysis between the different time points (days 7, 14, and 21) demonstrated that the approximately 30% lower dose of LCP-Tacro was associated with similar AUC24 (ratio of geometric means [RGM], day 14 vs. day 7: 97.49% and day 21 vs. day 7: 98.55%) and trough levels (Cmin; RGM, day 14 vs. day 7: 96.53% and day 21 vs. day 7: 97.23%) as Prograf but a lower peak (Cmax; RGM, day 14 vs. day 7: 71.59% and day 21 vs. day 7: 73.93%). Comparisons between days 21 and 14 demonstrated consistency for LCP-Tacro in tacrolimus concentration (AUC24; RGM, day 21 vs. day 14: 101.09%), peak levels (Cmax; RGM, day 21 vs. day 14: 103.28%), and trough levels (Cmin; RGM, day 21 vs. day 14: 100.72%) (Table 1).
TABLE 1.
Tacrolimus pharmacokinetic parameters
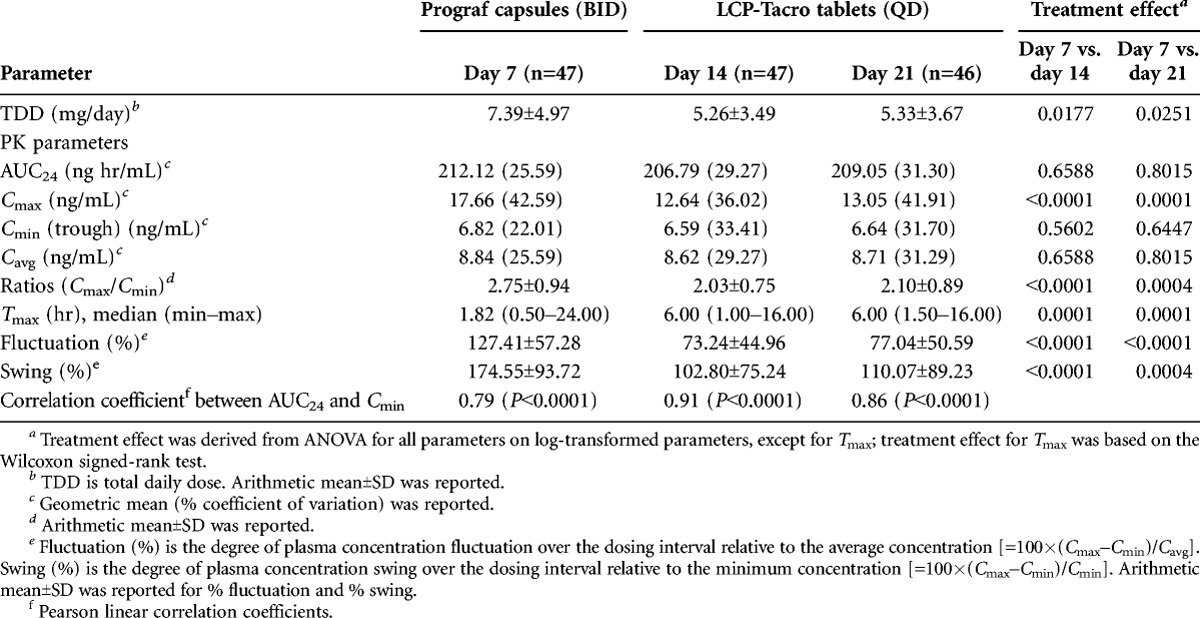
In terms of specific PK parameters, Cmax (P=0.0001), percent fluctuation (P<0.0001), and percent swing (P=0.0004) were significantly lower for LCP-Tacro tablets administered once-daily compared with twice-daily therapy with Prograf, whereas Tmax was significantly (P<0.001) longer (Table 1). Figure 2 shows the characteristic fluctuation in tacrolimus concentration (˜6–16 ng/mL) with Prograf and the comparative smaller fluctuation (˜7–12 ng/mL) with LCP-Tacro on days 14 and 21. AUC24 and Cmin were significantly correlated at each time point (days 7, 14, and 21) and the correlation coefficient between AUC24 and Cmin on days 14 and 21 (LCP-Tacro) were numerically higher than day 7 (Prograf), but the magnitude of the difference was not statistically significant (Table 1). Cmax, ratios of Cmax/Cmin, percent fluctuation, and percent swing were significantly (P≤0.0004) higher on day 7 (Prograf) versus day 14 (LCP-Tacro) and day 21 (LCP-Tacro).
FIGURE 2.
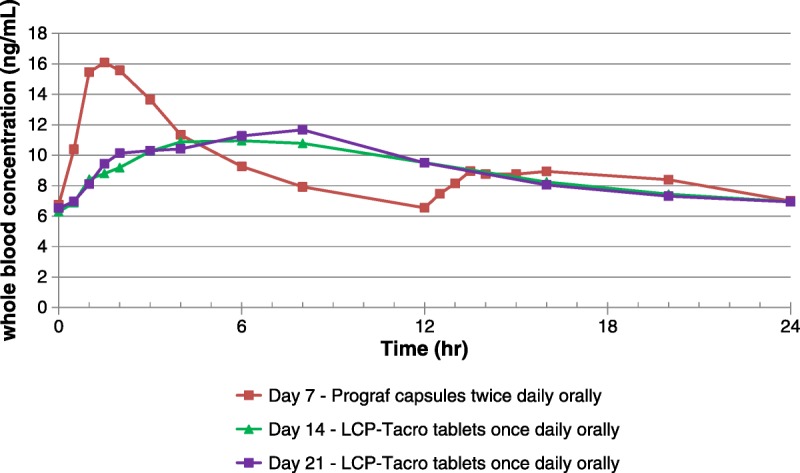
Mean whole-blood tacrolimus concentration in patients on days 7, 14, and 21 versus time.
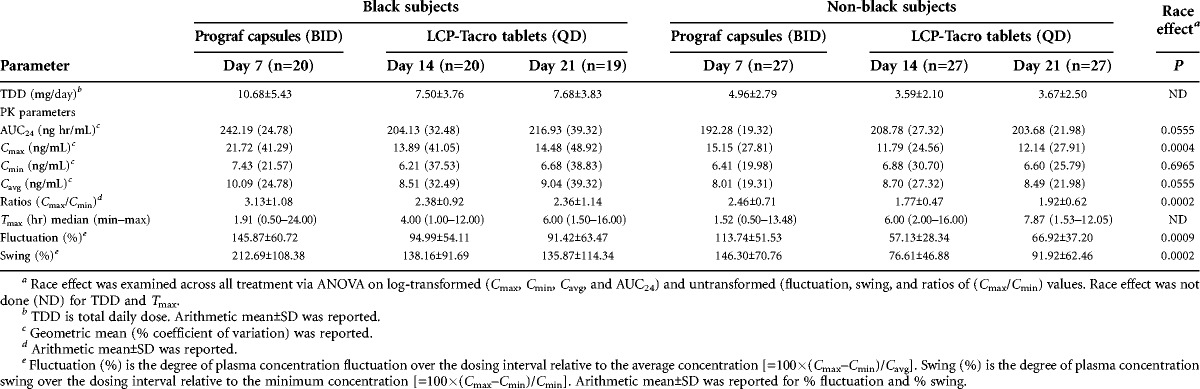
Black patients had a significantly higher mean Cmax (P=0.0004), degree of fluctuation (P=0.0009), and degree of swing (P=0.0002) than non-black patients; a nearly significant difference was seen for AUC24 (P=0.055). The differences between blacks and non-blacks in Cmax, percent fluctuation, and percent swing were seen with both Prograf and LCP-Tacro; however, the difference was consistently smaller with LCP-Tacro (Table 2).
TABLE 2.
Tacrolimus pharmacokinetic parameters—black and non-black patients
Safety
Extent of Exposure
The mean daily dose of tacrolimus for post-switch study periods of LCP-Tacro dosing was within the conversion range specified a priori targeting a switch factor of 0.70 (with actual ranging from 0.66 to 0.80 due to the nominal tablet dosage strengths of LCP-Tacro that were available). Trough levels were stable through the various study periods and within the 5 to 15 ng/mL range (median level ranged 7.3–8.9 ng/mL). If mean tacrolimus trough levels changed more than 25% from the mean tacrolimus trough levels from the initial 7 days of the study, the LCP-Tacro dose could be adjusted up or down by 25% on the morning of day 15. A total of 15 subjects had a dose adjustment for this reason.
Adverse Events
The overall incidence of AEs ranged from 8.3% to 29.4% across the study and follow-up periods. There were no meaningful differences between the treatments in the types or frequencies of AEs observed. A total of 54 AEs were reported; 12 events were ongoing at the time of study completion. Of those 12, 11 were mild events and 1 was moderate (anemia, unrelated to study drug). A total of three serious AEs were reported by three patients; none were considered to be related to treatment, and all resolved. One patient, while taking tacrolimus once-daily, was admitted to the hospital for angina; the patient continued taking tacrolimus once-daily and completed the study. A second patient was hospitalized for abdominal pain and nausea while taking tacrolimus twice-daily; the patient discontinued the study but remained taking tacrolimus twice-daily. A third patient was hospitalized for treatment of an acute febrile illness that occurred 2 days after the last dose of tacrolimus once-daily (day 23) at which time the patient was on tacrolimus twice-daily. Three patients discontinued due to AEs that were unrelated to treatment. No patients experienced graft loss or rejection. There were no deaths during the study.
Clinical Laboratory Values and Vital Signs
There were no clinically significant changes in clinical laboratory variables. Mean glucose was within normal range at day 21. Calculated glomerular filtration rates remained stable throughout the study (mean±SD glomerular filtration rate, screening: 58.67±16.845, day 0 [Prograf]: 58.93±14.650, day 7 [Prograf]: 59.62±16.372, day 14 [LCP-Tacro]: 57.21±14.134, and day 21 [LCP-Tacro]: 59.41±15.813).
There were no clinically significant changes in vital signs or electrocardiograms throughout the study.
DISCUSSION
In this phase 2 prospective study, adult stable kidney transplant recipients were safely converted from traditional Prograf capsules twice-daily to a fixed dose of once-daily LCP-Tacro tablet requiring approximately 30% less than the total daily dose of Prograf. The greater bioavailability of LCP-Tacro allows for once-daily dosing while achieving similar systemic exposure and trough levels. The PK profile of LCP-Tacro is characterized by flatter kinetics resulting in a steadier and more consistent concentration time profile over 24 hr and reduced peak-to-trough fluctuations and swing compared with Prograf. The robust correlation between AUC24 and Cmin with LCP-Tacro demonstrates that current practice of therapeutic drug monitoring of Cmin as a measure of tacrolimus exposure can also be applied to LCP-Tacro. In addition to the increased tacrolimus bioavailability associated with LCP-Tacro, the reduced peak-to-trough found for LCP-Tacro compared with Prograf and the excellent interday reproducibility in PK suggest a tighter and more consistent relationship between the dose given and the tacrolimus exposure level for LCP-Tacro compared with Prograf. This aspect has the potential to simplify the management of the kidney transplant patient and may result in less intrasubject variability and fewer dose changes, resulting in more time spent within targeted therapeutic range—a hypothesis that remains to be tested.
Importantly, the lower dosing benefit and favorable PK profile was seen for blacks who were converted from Prograf. The race effect differences were seen for both LCP-Tacro and Prograf, but the differences were less when treating with LCP-Tacro. As a result of the reduced bioavailability for blacks versus non-blacks seen in this study, subsequent clinical studies of LCP-Tacro employed a 0.85 conversion ratio when switching black patients from Prograf to LCP-Tacro. The PK differences between blacks and non-blacks seen in this study are consistent with the widely reported differences in tacrolimus absorption and metabolism reported from other studies. It is well documented that, largely due to a high frequency of the CYP3A5 genetic polymorphism that acts to increase clearance and lower oral bioavailability of tacrolimus, blacks require higher tacrolimus drug doses to achieve the same level of drug as non-blacks (9–15). Specifically, the wild-type gene, CYP3A5*1, which allows for significant production of CYP3A5, is reportedly absent in 60% to 90% non-blacks and yet present among 55% blacks. This may partially account for lower troughs and higher dose requirements of tacrolimus among blacks (16).
In this study, AEs were mild or moderate and did not significantly differ between the drug groups, and the incidence, type, and severity of AEs were in the range expected in this patient population. Most of the AEs were not related to study drug with no specific AE or unexpected trend of AEs that indicated a drug-related event. Overall, no new safety concerns related to LCP-Tacro were raised by the results of this study. Although most of the AEs were reported at the time patients were converted from Prograf to LCP-Tacro, this could reflect an increased awareness and reporting of AEs from clinicians due to the starting of a new medication. Importantly, the incidence of AEs decreased in study period 3 despite continued treatment. Laboratory values remained stable during the study.
The novel kinetic properties of LCP-Tacro enable a lower drug dose without affecting exposure, unlike published data from the modified-release version of tacrolimus currently approved by the European Medicines Agency (Advagraf), which show that higher doses are needed compared with Prograf to achieve therapeutic levels (17). It is plausible that a lower peak-to-trough fluctuation and swing may ultimately translate into less tacrolimus peak-related toxicity (8). Initial studies on tacrolimus revealed similar overall exposure (AUC) in patients experiencing neurotoxicity compared with patients without neurotoxicity and hypothesized that peak levels may correlate more closely to neurotoxicity (18). Studies designed to explicitly examine this hypothesis are warranted.
In addition to the benefits of the enhanced PK parameters associated with LCP-Tacro, the once-daily dosing of LCP-Tacro may also help to optimize tacrolimus therapy in kidney transplantation. Maintaining effective immunosuppressive drug levels is essential to preventing rejection and lack of adherence to prescribed immunosuppression drug regimens is a barrier to successful transplant outcomes (5, 19). Unfortunately, lack of adherence has been reported to be common in transplant recipients (20–22). Greater dose frequency is inversely related to medication adherence (23–26). In a review of medication adherence in chronic conditions, the most effective interventions appear to be those that simplify dosing demands (27, 28). Thus, the once-daily dosing afforded by LCP-Tacro may be associated increased medication adherence. Well-designed studies are needed to evaluate this potential advantage of once-daily LCP-Tacro.
Results from this study demonstrated that stable kidney transplant patients could be converted to LCP-Tacro tablets at a mean conversion ratio of 0.71, that is, a fixed dose approximately 30% less than the total daily dose of Prograf. LCP-Tacro showed greater bioavailability and a less peak-to-trough fluctuations at steady state, overall flatter kinetics compared with Prograf.
MATERIALS AND METHODS
Study Design and Conduct
This was a phase 2, open-label, multicenter, prospective U.S. study of adult stable kidney transplant patients who were converted from Prograf capsules twice-daily to LCP-Tacro tablets once-daily.
Following study enrolment, each patient was monitored for 7 days on a fixed dose of Prograf capsules twice-daily to ensure stable tacrolimus trough concentrations between 7 and 12 ng/mL. On day 7, a 24-hr PK assessment was conducted for each patient. On day 8, each patient was converted to LCP-Tacro tablets once-daily using a dose conversion ratio targeting 0.70 and ranging from 0.66 to 0.80 (due to the nominal dosage strengths of LCP-Tacro that were available); patients continued on a fixed dose of LCP-Tacro for the second week of the study (days 8–14). Tacrolimus trough blood levels (Cmin) were obtained once between days 9 and 11 and once between days 11 and 13, with at least 48 hr between the two measurements to assure maintenance of tacrolimus blood concentrations between 5 and 15 ng/mL. On days 14 and 21, a 24-hr PK assessment was conducted. If the patient’s trough levels were maintained between 5 and 15 ng/mL during study days 8 to 14 without any need for change in the study drug dose, the patient proceeded to study days 15 to 21 on the same fixed dose of LCP-Tacro tablets once-daily. Tacrolimus trough blood levels were obtained once between days 16 and 18 and once between days 18 and 20, with at least 48 hr between the two measurements. On the morning of day 22, the patient was converted back to their regular maintenance regimen of Prograf capsules twice-daily. A follow-up safety visit was conducted on day 52 (Fig. 3).
FIGURE 3.
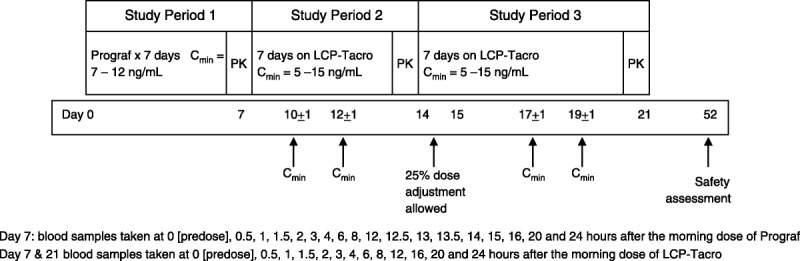
Study design.
Participants
All participants provided written consent for participation; this study was conducted in accordance with the Helsinki Declaration of 1975.
Inclusion and Exclusion Criteria
Eligible participants were adult (ages 18–65 years) kidney transplant recipients at least 6 months after transplantation, with serum creatinine 2.0 mg/dL or more before enrolment, on oral Prograf therapy as part of their maintenance immunosuppression therapy. The Prograf dose must have been stable with tacrolimus trough levels between 7 and 12 ng/mL for 2 weeks or more before enrolment. Patients could continue to take mycophenolate mofetil (CellCept; Roche Laboratories, Nutley, NJ) or mycophenolic acid delayed-release tablets (Myfortic; Novartis Pharmaceuticals, East Hanover, NJ), provided the doses had been stable for 2 weeks or more before enrolment. Exclusion criteria included receipt of any transplanted organ other than the kidney, white blood count 2.8×109/L or less, total Prograf dose for 24 hr of less than 3 mg, taking any drug interfering with tacrolimus metabolism, had taken sirolimus within 3 months before screening, had acute rejection requiring antibody therapy within 6 months before enrolment, had been treated for acute rejection within 30 days before enrolment, or any gastrointestinal disorder that may have affected the absorption of tacrolimus.
Primary Objective
The primary objective of the study was to demonstrate the steady-state tacrolimus exposure (AUC24) and trough levels (Cmin) in stable kidney transplant recipients converted from Prograf to LCP-Tacro.
Secondary Objectives
The secondary objectives of the study were to evaluate tacrolimus exposure and trough concentrations in stable kidney transplant recipients converted from Prograf to LCP-Tacro, to determine the mean conversion ratio between Prograf capsules twice-daily and LCP-Tacro tablets once-daily, and to evaluate the safety of LCP-Tacro compared with Prograf. Safety was evaluated by monitoring the AEs, serious AEs, vital signs, clinical laboratory test results, physical examinations, and electrocardiograms.
Sample Size Determination
The sample size was calculated based on the endpoint of equivalence of natural logarithm-transformed AUC24 using the following assumptions: two, one-sided tests; equivalence limits of 80% to 125%, and common standard deviation of 0.220. If the sample in each group was 18, a two-group design would have 80% power to reject the null hypothesis that the test mean and standard mean were not equivalent. For this one-sample study, the estimated sample size needed per group was doubled to get the best estimate for the true sample size needed; taking into consideration a dropout rate of approximately 30%, the total estimated patient population was 50.
Statistical Analysis
All demographic data, PK parameters, laboratory data, and AEs were summarized using descriptive statistics.
PK parameters (AUC24, Cmax, Cmin, Cavg, and Tmax) were calculated from blood concentration–time data via noncompartmental analysis. Analysis of variance (ANOVA) was performed on natural log-transformed PK parameters and on untransformed parameters percent fluctuation and percent swing and Cmax/Cmin. The model included treatment as a factor. The ratio of geometric least squares means and 90% confidence intervals were calculated on the following three comparisons for AUC24, Cmax, and Cmin: day 14 LCP-Tacro versus day 7 Prograf, day 21 LCP-Tacro versus day 7 Prograf, and day 21 LCP-Tacro versus day 14 LCP-Tacro.
Pairwise treatment comparisons were made for Tmax using Wilcoxon signed-rank test. The mean shift between two treatments was estimated by the median unbiased Hodges-Lehmann estimate. The degree of correlation between AUC24 and Cmin was quantified by calculating a Pearson correlation coefficient; both parameters were natural log-transformed before correlation analysis. Analysis of subgroups was performed by race (black vs. non-black). Difference between race groups in each parameter was analyzed via one-way ANOVA.
Analysis Populations
The intent-to-treat (ITT) population included all patients who fulfilled all inclusion and exclusion criteria and were enrolled in the study. The per-protocol population included the subset of the ITT population who completed the study without any major deviations from the protocol procedures. All safety evaluations were based on the ITT population; all PK analyses were based on the per-protocol population.
ACKNOWLEDGMENTS
Investigators: Goran Klintmalm, M.D. (Baylor University Medical Center, Dallas, TX); Kimi R. Ueda, Pharm.D. (California Pacific Medical Center, San Francisco, CA); Rita Alloway, Pharm.D. (University of Cincinnati Medical Center & The Christ Hospital, Cincinnati, OH); Shamkant Mulgaonkar, M.D. (St. Barnabas Healthcare System, Livingston, NJ); Suphamai Bunnapradist, M.D. (University of California, Los Angeles, CA); Robert Gaston, M.D. (University of Alabama at Birmingham, AL); Ken Brayman, M.D. (University of Virginia, Charlottesville, VA); A. Osama Gaber, M.D. (The Methodist Hospital, Houston, TX); Richard Freeman, M.D. (Tufts New England Medical Center, Boston, MA); Reginald Y. Gohh, M.D. (Rhode Island Hospital, Providence, RI); Kenneth Bodziak, M.D. (University Hospitals Case Medical Center, Cleveland, OH); Bruce Kaplan, M.D. (University of Illinois, Chicago, IL); and Michael Germain, M.D. (Western New England Renal Transplant Associates, Springfield, MA). Kristin Kistler, Ph.D., from United Biosource Corp. provided medical writing support.
Footnotes
Financial support for this study was provided by Veloxis Pharmaceuticals to United Biosource Corp. for article writing support.
Portions of these data were presented at the 2008 American Transplant Congress in Toronto and the 2012 American Transplant Congress in Boston.
ClinicalTrials.gov identifier: NCT00496483.
Each author was a study investigator. No financial support was provided to the authors for the article writing. The authors declare no conflicts of interest.
E-mail: aogaber@tmhs.org
A.O.G., R.R.A., K.B., B.K., and S.B. participated in the interpretation of data, writing of the initial drafts of the article, critical revision of the article, and approval of the submitted and final versions.
Received 26 February 2013. Revision requested 4 March 2013.
Accepted 8 April 2013.
REFERENCES
- 1. Matas AJ, Smith JM, Skeans MA, et al. Special issue: Organ Procurement and Transplantation Network and Scientific Registry of Transplant Recipients 2011 data report. OPTN/SRTR 2011 annual data report: kidney. Am J Transplant 2013; 13: 1123237695 [Google Scholar]
- 2. Webster AC, Taylor RRS, Chapman JR, et al. Tacrolimus versus cyclosporin as primary immunosuppression for kidney transplant recipients. Cochrane Database Syst Rev 2005; CD003961 [DOI] [PubMed] [Google Scholar]
- 3. Wallemacq P, Armstrong VW, Brunet M, et al. Opportunities to optimize tacrolimus therapy in solid organ transplantation: report of the European consensus conference. Ther Drug Monit 2009; 31: 139. [DOI] [PubMed] [Google Scholar]
- 4.Prograf Prescribing Information (Astellas Pharma US, Inc., Deerfield, IL, July 2011). [Google Scholar]
- 5. Morrissey PE, Reinert S, Yango A, et al. Factors contributing to acute rejection in renal transplantation: the role of noncompliance. Transplant Proc 2005; 37: 2044. [DOI] [PubMed] [Google Scholar]
- 6.FDA Bioavailability and Bioequivalence Studies for Orally Administered Drug Products—General Considerations: Guidance for Industry. Rockville: FDA Center for Drug Evaluation and Research; 2003 [Google Scholar]
- 7. Endrenyi L, Tothfalusi L. Metrics for the evaluation of bioequivalence of modified-release formulations. AAPS J 2012; 14: 813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.EMEA Note for Guidance on Modified Release Oral and Transdermal Dosage Forms: Section II (Pharmacokinetic and Clinical Evaluation). London: European Agency for the Evaluation of Medicinal Products, Committee for Proprietary Medicinal Products; 1999 [Google Scholar]
- 9. Jacobson PA, Oetting WS, Brearley AM, et al. Novel polymorphisms associated with tacrolimus trough concentrations: results from a multicenter kidney transplant consortium. Transplantation 2011; 91: 300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Staatz CE, Goodman LK, Tett SE. Effect of CYP3A and ABCB1 single nucleotide polymorphisms on the pharmacokinetics and pharmacodynamics of calcineurin inhibitors: part I. Clin Pharmacokinet 2010; 49: 141. [DOI] [PubMed] [Google Scholar]
- 11. Staatz CE, Goodman LK, Tett SE. Effect of CYP3A and ABCB1 single nucleotide polymorphisms on the pharmacokinetics and pharmacodynamics of calcineurin inhibitors: part II. Clin Pharmacokinet 2010; 49: 207. [DOI] [PubMed] [Google Scholar]
- 12. Op den Buijsch RAM, Christiaans MHL, Stolk LML, et al. Tacrolimus pharmacokinetics and pharmacogenetics: influence of adenosine triphosphate-binding cassette B1 (ABCB1) and cytochrome (CYP) 3A polymorphisms. Fundam Clin Pharmacol 2007; 21: 427. [DOI] [PubMed] [Google Scholar]
- 13. Quteineh L, Verstuyft C, Furlan V, et al. Influence of CYP3A5 genetic polymorphism on tacrolimus daily dose requirements and acute rejection in renal graft recipients. Basic Clin Pharmacol Toxicol 2008; 103: 546. [DOI] [PubMed] [Google Scholar]
- 14. Macphee IAM, Fredericks S, Tai T, et al. Tacrolimus pharmacogenetics: polymorphisms associated with expression of cytochrome P4503A5 and P-glycoprotein correlate with dose requirement. Transplantation 2002; 74: 1486. [DOI] [PubMed] [Google Scholar]
- 15. Dirks NL, Huth B, Yates CR, et al. Pharmacokinetics of immunosuppressants: a perspective on ethnic differences. Int J Clin Pharmacol Ther 2004; 42: 701. [DOI] [PubMed] [Google Scholar]
- 16. Lamba JK, Lin YS, Shuetz EG, et al. Genetic contribution to variable human CYP3A-mediated metabolism. Adv Drug Deliv Rev 2002; 54: 1271. [DOI] [PubMed] [Google Scholar]
- 17. de Jonge H, Kuypers DR, Verbeke K, et al. Reduced C0 concentrations and increased dose requirements in renal allograft recipients converted to the novel once-daily tacrolimus formulation. Transplantation 2010; 90: 523. [DOI] [PubMed] [Google Scholar]
- 18. Wijdicks EFM, Wiesner RH, Dahlke LJ, et al. FK5 06-induced neurotoxicity in liver transplantation. Ann Neurol 1994; 35: 498. [DOI] [PubMed] [Google Scholar]
- 19. Michelon T, Dominguez V, Losekan A, et al. Kidney graft failure due to noncompliance. Transplant Proc 1999; 31: 3031. [DOI] [PubMed] [Google Scholar]
- 20. Germani G, Lazzaro S, Gnoato F, et al. Nonadherent behaviors after solid organ transplantation. Transplant Proc 2011; 43: 318. [DOI] [PubMed] [Google Scholar]
- 21. Chisholm MA. Issues of adherence to immunosuppressant therapy after solid-organ transplantation. Drugs 2002; 62: 567. [DOI] [PubMed] [Google Scholar]
- 22. Laederach-Hofmann K, Bunzel B. Noncompliance in organ transplant recipients: a literature review. Gen Hosp Psychiatry 2000; 22: 412. [DOI] [PubMed] [Google Scholar]
- 23. Eisen SA, Miller DK, Woodward RS, et al. The effect of prescribed daily dose frequency on patient medication compliance. Arch Intern Med 1990; 150: 1881. [PubMed] [Google Scholar]
- 24. Claxton AJ, Cramer J, Pierce C. A systematic review of the associations between dose regimens and medication compliance. Clin Ther 2001; 23: 1296. [DOI] [PubMed] [Google Scholar]
- 25. Saini SD, Schoenfeld P, Kaulback K, et al. Effect of medication dosing frequency on adherence in chronic diseases. Am J Manag Care 2009; 15: e22. [PubMed] [Google Scholar]
- 26. Feldman HI, Hackett M, Bilker W, et al. Potential utility of electronic drug compliance monitoring in measures of adverse outcomes associated with immunosuppressive agents. Pharmacoepidemiol Drug Saf 1999; 8: 1. [DOI] [PubMed] [Google Scholar]
- 27. Kripalani S, Yao X, Haynes RB. Interventions to enhance medication adherence in chronic medical conditions: a systematic review. Arch Intern Med 2007; 167: 540. [DOI] [PubMed] [Google Scholar]
- 28. Morales JM, Varo E, Lazaro P. Immunosuppressant treatment adherence, barriers to adherence and quality of life in renal and liver transplant recipients in Spain. Clin Transplant 2012; 26: 369. [DOI] [PubMed] [Google Scholar]


