Abstract
Activation-induced cytidine deaminase (AID) plays a key role in B cell immunoglobulin (Ig) class switch recombination (CSR) and somatic hypermutation (SHM). We have previously reported that the highly conserved homeodomain HoxC4 transcription factor binds to the Aicda (AID gene) promoter to induce AID expression. Here, we investigated the regulation of HoxC4 transcription by a proliferation-inducing ligand (APRIL) and B cell-activating factor belonging to the TNF family (BAFF) in mouse B cells. APRIL substantially increased both HoxC4 and AID expression, whereas BAFF induced the expression of AID but not HoxC4. To elucidate the underlying mechanisms, we constructed a HoxC4 gene promoter reporter vector and analyzed the promoter induction after APRIL stimulation. APRIL enhanced the HoxC4 promoter activity by 2.3-fold, and this increase disappeared when the second putative NF-κB-binding promoter element (NBE2) was mutated. Based on ChIP assays, we found that NF-κB bound to the HoxC4 promoter NBE2 region. Furthermore, the overexpression of NF-κB augmented the APRIL-induced HoxC4 promoter activity, while the expression of dominant negative-IkBa suppressed it. Taken together, our findings suggest that NF-κB mediates APRIL-induced HoxC4 transcription.
Keywords: HoxC4, APRIL, AID, NF-κB, B cells
Introduction
Immunoglobulin (Ig) class switch recombination (CSR) and somatic hypermutation (SHM) are critical molecular mechanisms that endow antibody molecules with higher diversity and broader biological effector functions. Both CSR and SHM require the enzyme activation-induced cytidine deaminase (AID), which is highly expressed in activated germinal center (GC) B cells during immune responses [1,2]. We have shown that AID transcription is significantly increased by B cells stimulated with LPS, CD40 ligand (CD154), and IL-4 [1,2]. In mouse and human B cells, CD154 and IL-4 induce AID expression through NF-κB and Stat6, respectively. We have also shown that Stat6 and PKA/CREB are involved in IL-4-induced AID expression [3]. Other transcription factors, such as E-protein (E47) [4], Pax5 [5], IRF-8 [6], and Sp1/3 [7], are known to regulate the Aicda (AID gene) locus to induce AID transcription [2,8–10]. HoxC4 binds to a highly conserved 5'-ATTTGAAT-3' motif in the Aicda promoter and activates this promoter for AID expression leading to CSR and SHM [11]. HoxC4 is expressed in GC B cells and HoxC4 expression is specifically upregulated by the same stimuli that induce AID expression (LPS, CD154 and IL-4) [11–13]. HoxC4 is further upregulated by estrogen-estrogen receptor complexes, which bind to three highly conserved estrogen response elements (EREs) that we identified in the HoxC4 promoter [9]. Thus, estrogen upregulates AID expression through the induction of HoxC4, which likely contributes to the high rates of antibody CSR and SHM in autoimmune conditions with a high prevalence in females, such as systemic lupus erythematosus (SLE).
The TNF superfamily members 13 and 13b, a proliferation-inducing ligand (APRIL) and B cell-activating factor belonging to the TNF family (BAFF), induce AID expression and Ig CSR [14,15]. We have demonstrated that macrophage-derived BAFF stimulates mouse B cells to express AID through the p38/MAPK/CREB and JNK/AP-1 signaling pathways [16]. Whether APRIL and/or BAFF upregulate HoxC4 to induce AID expression has not been addressed. In this study, we investigated the effects of APRIL and BAFF on the transcriptional regulation of HoxC4 in B cells. We found that APRIL induces HoxC4 transcription by enabling NF-κB binding to the HoxC4 promoter.
Materials and methods
Animals
BALB/c mice were purchased from Damool Science (Daejeon, Korea) and maintained on an 8:16 h light:dark cycle in an animal environmental control chamber. Eight- to twelve-week-old mice were used, and animal care was conducted in accordance with the institutional guidelines of the Institutional Animal Care and Use Committee of Konyang University.
Cell culture and reagents
The mouse B lymphoma cell line CH12F3-2A (surface, Igμ+) [17] was kindly provided by Dr. T. Honjo (Kyoto University, Kyoto, Japan). Mouse spleen B cell suspensions were prepared as described previously [18]. The cells were cultured at 37°C in a humidified CO2 incubator (Forma Scientific, Marietta, OH, USA) in RPMI-1640 medium (WelGENE, Daegu, Korea) supplemented with 10% fetal bovine serum (PAA Laboratories, Etobicoke, ON, Canada). The cells were stimulated with LPS (0.5 μg/ml for spleen B cells or 12.5 μg/ml for CH12F3-2A, E. coli 0111:B4; Sigma, St. Louis, MO, USA), IL-4 (10 ng/ml, R&D Systems, Minneapolis, MN, USA), BAFF (100 ng/ml, R&D Systems), and APRIL (100 ng/ml, R&D Systems). The p38 inhibitor (SB203580) and the JNK inhibitor (SP600125) were purchased from Selleck Chemicals (Houston, TX, USA).
Expression and reporter plasmids
The NF-κB p50, p65, and p50–p65 fusion proteins that were subcloned into pcDNA3 [19,20] were provided by Dr. J. Stavnezer (University of Massachusetts Medical School, Worcester, MA, USA). The expression plasmid for dominant negative (DN)-IκBα (3C/2N-IκBα) [21] was obtained from Dr. M. Arsura (University of Tennessee Cancer Institute, Memphis, TN, USA). The HoxC4 promoter DNA fragment (−521 to +45; HR2C1) was amplified from mouse kidney genomic DNA by PCR using the following primers: forward 5'-CGGCTCGAGGCTCTGGGGCCTGGGCCGGG-3' and reverse 5'-CGGAAGCTTTCCTTCCTTGCTAAAGATGG-3'. The HoxC4 promoter segment was subcloned into pGL3-Enhancer Vector (Promega, Madison, WI, USA), and the promoter reporter was named pGL3e-HR2C1. pGL3e-HR2C1 reporters containing mutations in the putative NBEs were constructed using the QuikChange™ Site-Directed Mutagenesis method (Stratagene, La Jolla, CA, USA).
RT-PCR
RNA preparation, reverse transcription, and PCR were performed as described previously [22]. The following PCR primers were synthesized by Bioneer (Seoul, Korea): AID, forward 5'-AGATAGTGCCACCTCCTGCTCACTGG-3', reverse 5'-GGCTGAGGTTAGGGTTCCATCTCAG-3' (product size, 209 bp); HoxC4, forward 5'-CTACCTGACCCGAAGGAGAA-3', reverse 5'-TGACCTCACTTTGGTGTTGG-3' (product size, 136 bp); and β-actin, forward 5'-CATGTTTGAGACCTTCAACACCCC-3', reverse 5'-GCCATCTCCTGCTCGAAGTCTAG-3' (product size, 320 bp). All reagents for RT-PCR were purchased from Promega. PCR for β-actin was performed in parallel to normalize cDNA concentrations within each set of samples. Aliquots of the PCR products were resolved by electrophoresis on 2% agarose gels.
Transfection and luciferase reporter assays
Transfection was performed by electroporation with a Gene Pulser II (Bio-Rad, Hercules, CA, USA) [22]. Reporter plasmids were co-transfected with expression plasmids and pCMVβgal (Stratagene), and luciferase and β-gal assays were performed as described previously [22].
Chromatin immunoprecipitation assay
Chromatin immunoprecipitation (ChIP) assays were performed using a ChIP assay kit (Upstate Biotechnology, Inc., Lake Placid, NY, USA). Cells (3×107) were fixed with 1% formaldehyde, washed, resuspended in lysis buffer, and sonicated. After removing cell debris by centrifugation, the supernatant was diluted ten-fold with ChIP dilution buffer and precleared with a salmon sperm DNA/Protein A agarose-50% slurry. The supernatant fraction was transferred to a fresh tube with 10 μg/ml anti-NF-κB p65 (C-20) and anti-NF-κB p50 (H-119) rabbit polyclonal antibodies (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and incubated overnight at 4°C. Salmon sperm DNA/Protein A agarose-50% slurry (60 μl) was added to the immune complexes, and after incubation for 1 h at 4°C, the supernatant was discarded. The protein-DNA cross-links were reversed by digestion with 2 μl of 10 mg/ml proteinase K, and the DNA was extracted, dissolved in 20 μl Tris/EDTA buffer, and subjected to PCR. The primer sequences for the HoxC4 promoter region were as follows: forward, 5'-CATCCAACCTGCACTGAAGC-3', and reverse, 5'-AGTGCCTTTCCAGTGGTCTC-3' (product size, 119 bp). The PCR products were resolved by electrophoresis on 2% agarose gels.
Statistical analysis
Statistical differences between experimental groups were determined by analysis of variances. Values with p < 0.05 by an unpaired two-tailed Student's t test were considered significant.
Results and discussion
APRIL induces HoxC4 expression in mouse B cells
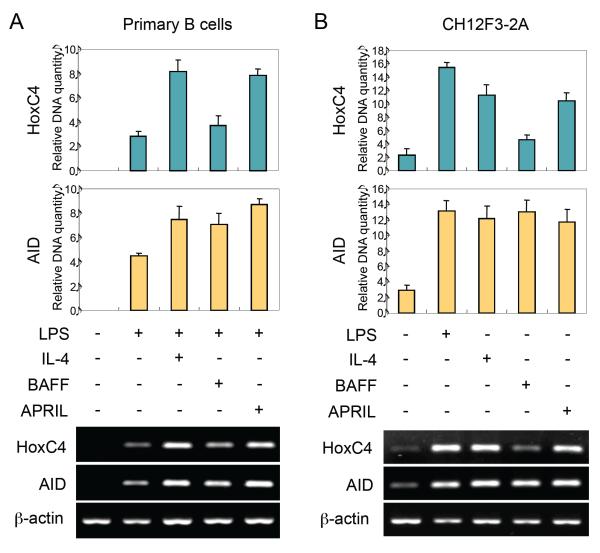
APRIL and BAFF have been shown to induce Aicda mRNA expression in mouse B cells [14,15]. As we have shown, HoxC4 is a critical transcription factor for Aicda promoter activation, and it mediates Aicda transcription in response to a variety of stimuli, including estrogen hormones, which play a significant role in AID upregulation in lupus-prone female mice and possibly in lupus patients [9,11,23]. We first determined whether APRIL and BAFF also induce HoxC4 mRNA expression. As we have reported [9,11], LPS and IL-4 induced HoxC4 and AID expression in mouse B cells. APRIL also induced HoxC4 and AID mRNA expression, while BAFF induced AID but not HoxC4 (Fig. 1A and B). These results support the hypothesis that APRIL induces AID expression through HoxC4 enhancement.
Fig. 1.
Effects of APRIL and BAFF on the expression of HoxC4 and AID. Mouse spleen B cells (A) or CH12F3-2A cells (B) were stimulated with LPS, IL-4, BAFF, or APRIL, as indicated. After 48 h of culture, RNAs were isolated, and levels of HoxC4 and AID mRNA were measured by RT-PCR. The graphs show relative HoxC4 and AID cDNA levels normalized to the expression of β-actin cDNA by ImageJ (NIH, Bethesda, MD, USA) analysis. Densitometric data are averages of two independent experiments with ranges (bars).
Effect of APRIL on HoxC4 promoter activity
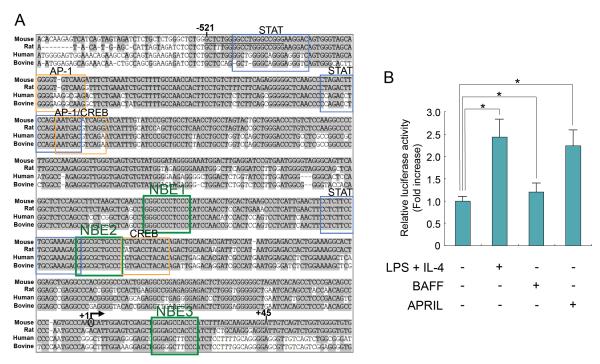
We next surveyed the 5' flanking DNA of the HoxC4 gene for sequences containing potential transcription factor-binding elements in four species. We then constructed a mouse HoxC4 gene promoter reporter vector to analyze the induction of HoxC4 transcriptional activation by APRIL and BAFF. We identified multiple putative NF-κB-binding elements (NBE1, NBE2, NBE3) as well as STAT-, AP1-, and CREB-binding sites within the HoxC4 promoter region (Fig. 2A) (TFSEARCH Version 1.3, Parallel Application TRC Laboratory, RWCP, Tokyo, Japan; MatInspector Version 3.0, Genomatix Software GmbH, Munich, Germany; MATCH™ public version 1.0, BIOBASE GmbH, Wolfenbuttel, Germany). Consistent with our endogenous HoxC4 mRNA expression findings (Fig. 1), BAFF did not significantly induce HoxC4 promoter activation. By contrast, APRIL induced HoxC4 promoter activation by 2.3-fold (Fig. 2B). Thus, APRIL effectively activates the HoxC4 promoter to induce HoxC4 and subsequent AID expression.
Fig. 2.
Effect of APRIL on HoxC4 promoter activity. (A) Alignment of the 5' flanking DNA sequences of the HoxC4 gene in mouse, rat, human, and bovine. Gray shading indicates DNA sequences conserved among the four species. The putative STAT, AP1, and CREB binding sites as well as NF-κB binding elements (NBE1, NBE2, NBE3) were boxed. The region utilized for the HoxC4 promoter luciferase reporter construct (pGL3e-HR2C1) is indicated by vertical bars (−521, +45). The arrow marks the putative transcription initiation site (+1) of the mouse HoxC4 gene (GeneID: 15423). (B) CH12F3-2A cells were transfected with the pGL3e-HR2C1 reporter (15 μg). Cells were then stimulated with LPS, IL-4, BAFF, or APRIL, and luciferase activity was assayed 16 h later. The transfection efficiency was normalized to β-gal activity. Data represent the average luciferase activity from three independent transfections with SEM error bars. *p < 0.05.
APRIL-induced activation of HoxC4 promoter is mediated by NF-κB
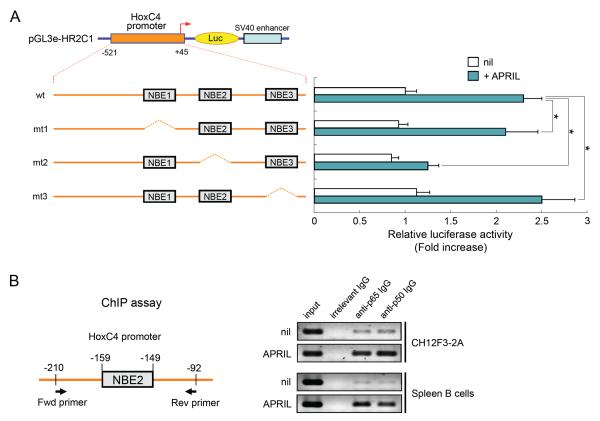
The activation of NF-κB represents one of the most important APRIL-induced mechanisms in the B cell signaling cascade [24]. To determine whether the three putative NF-κB-binding elements (NBEs), which were identified within the HoxC4 promoter (Fig. 2A), are important for the activation of this gene, we tested the effects of the deletion of each of the NBE using transient reporter assays in CH12F3-2A cells. The mutant reporters (mt1 and mt3) responded to APRIL similar to the wild-type (wt) pGL3e-HR2C1 (Fig. 3A). However, under similar conditions, the reporter activity of mt2 was significantly reduced, indicating that the second NBE (NBE2) is required for APRIL-induced promoter activation.
Fig. 3.
Functional analysis of putative NF-κB binding sites in HoxC4 promoter activity. (A) Effect of APRIL on the promoter activity of mutant HoxC4 promoter reporters. Three different mutant reporters were constructed (mt1, mt2, mt3), which lacked each NBE, as indicated. CH12F3-2A cells were transfected with the reporters (15 μg). APRIL was added, and the luciferase activity was determined 16 h later. Transfection efficiency was normalized to β-gal activity. Data shown are the average luciferase activity of three independent transfections with SEM (bars). *p < 0.05. (B) ChIP assay to determine whether NF-κB bound to the HoxC4 promoter region (NBE2). PCR of crosslinked chromatin precipitated from APRIL-stimulated or -nonstimulated (nil) CH12F3-2A and mouse spleen B cells with preimmune control (irrelevant rabbit IgG) or anti-p65 or anti-p50 Ab that were amplified for the HoxC4 promoter with the indicated primers.
APRIL can also activate the JNK and p38 pathways [25], which eventually activate the transcription factors AP-1 and CREB. Thus, we constructed deletion mutant reporters for putative AP-1 and CREB binding sites (Fig. 2A) in pGL3e-HR2C1 and measured the activation of these HoxC4 promoter constructs. We did not detect any decrease in HoxC4 promoter activation in AP-1 or CREB binding site deletion mutant reporters compared to the wt reporter (data not shown). In addition, treatment with either a p38 inhibitor (SB203580) or a JNK inhibitor (SP600125) did not affect APRIL-induced HoxC4 mRNA expression or APRIL-induced HoxC4 promoter activation in both mouse spleen B cells and CH12F3-2A cells (data not shown). This further suggests that, in B cells, APRIL induces HoxC4 transcription through the NF-κB pathway but not through the JNK/p38 pathway.
Next, we used a chromatin immunoprecipitation (ChIP) assay to investigate the binding of NF-κB proteins to the NBE2 region of the HoxC4 promoter in APRIL-treated CH12F3-2A B cells or primary mouse B cells. We specified the HoxC4 promoter DNA sequence containing NBE2 in the DNA that was precipitated by Ab to p65 or p50 from either CH12F3-2A or primary mouse B cells. The amount of the specified HoxC4 DNA promoter was further increased by APRIL stimulation, suggesting an increased recruitment of NF-κB proteins to this promoter (Fig. 3B). Thus, our results indicate that NF-κB is induced by APRIL signaling and binds to NBE2 of the HoxC4 promoter.
Overexpression of NF-κB enhances HoxC4 promoter activation
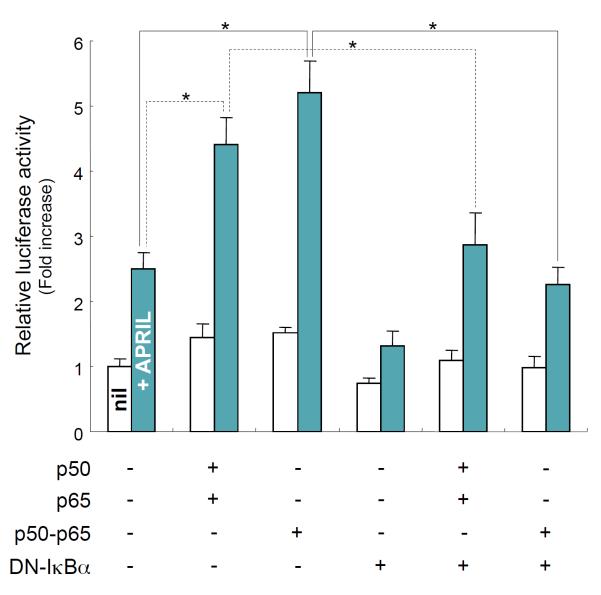
Because our results indicate that NF-κB is a critical transcriptional activator in APRIL-induced HoxC4 promoter activation, we assessed the effect of NF-κB on HoxC4 promoter activation. The overexpression of the p50 and p65 subunits of NF-κB and a p50–p65 fusion gene increased APRIL-induced promoter activation by 1.8- and 2.1-fold, respectively (Fig. 4). Additionally, this increase was abolished by the overexpression of a dominant negative (DN) form of IκBα (DN-IκBα), an active inhibitor of NF-κB. These experiments further emphasize the role of NF-κB in mediating APRIL-induced HoxC4 promoter activation. Thus, NF-κB plays an important role in HoxC4 transcription. On the other hand, it has been known for many years that NF-κB is a redox-sensitive transcription factor [26,27]. Hence, there is a good chance that HoxC4 expression might be up-regulated by oxidative stress through a NF-κB-mediated mechanism in mouse B cells. However, this possibility remains to be tested.
Fig. 4.
Effect of NF-κB overexpression on APRIL-induced HoxC4 promoter activity. CH12F3-2A cells were transfected with reporters (15 μg) and the indicated expression plasmids (10 μg). APRIL was added, and luciferase activity determined 16 h later. Transfection efficiency was normalized to β-gal activities. Data shown are average luciferase activities of three independent transfections with SEM (bars). *p < 0.05.
Overall, these findings provide new evidence that APRIL, a cytokine important in B cell proliferation and differentiation, induces HoxC4 expression through NF-κB, which binds to and activates the HoxC4 promoter to induce HoxC4 transcription. The HoxC4 protein in turn activates the Aicda promoter to induce AID expression. In spite of our unequivocal demonstration of a role for HoxC4 in APRIL-induced AID upregulation, we cannot rule out the possibility that APRIL triggers signaling pathways that directly induce AID expression. Indeed, we are currently exploring the direct regulation of AID promoter activity by APRIL.
APRIL and BAFF are produced by monocytes, macrophages, and dendritic cells and share two common receptors: the transmembrane activator, calcium modulator, and cyclophilin ligand interactor (TACI) and B-cell maturation antigen (BCMA), which are both expressed on B cells [28,29]. Through TACI and BCMA, both APRIL and BAFF provide critical signals for the survival and proliferation of peripheral B cells and plasma cells [30] and both can induce AID and promote Ig CSR [14,15,31]. Despite these structural and functional similarities, APRIL and BAFF also display different activities. As we have shown here, unlike APRIL, BAFF could not induce HoxC4 mRNA expression and promoter activation (Fig. 1 and 2B). Moreover, APRIL, but not BAFF, binds to heparin sulfate proteoglycans (HSPGs) as receptors [32,33]. This different receptor usage may explain the efficient induction of HoxC4 expression by APRIL but not BAFF. Thus, it will be important to investigate the receptor(s) through which APRIL induces HoxC4 and subsequent AID expression in B cells. Like APRIL, BAFF induces NF-κB activation in mouse B cells [24]. However, BAFF did not induce HoxC4 promoter activation (Fig. 2B), although an important NF-κB binding element (NBE3) is located in the promoter (Fig. 3). The underlying mechanisms of the inability of BAFF for HoxC4 induction involving NF-κB remain to be determined. There are two possibilities which will explain the difference between APRIL and BAFF to induce HoxC4 expression even if both cytokines can activate NF-κB. The first is that different NF-κB family members might be activated by the two different cytokines and only certain kinds of activated NF-κB are involved in HoxC4 expression. The second possibility is that BAFF-activated NF-κB may not bind to the NBE of HoxC4 promoter because the NBE is a composite binding site for only a distinct set of APRIL-activated NF-κB. In fact, we have recently shown that NF-κB does not mediate BAFF-induced AID expression in mouse B cells [16]. Since HoxC4 directly induces AID expression, this result strongly suggests that BAFF-induced, NF-κB-mediated transcriptional activation does not involved in HoxC4 gene promoter.
High levels of APRIL and BAFF recur in patients with autoimmune diseases, such as systemic lupus, rheumatoid arthritis, multiple sclerosis, and Sjögren's syndrome, and correlate with pathogenic autoantibody titers [29]. As we have shown, HoxC4 plays a major role in mediating AID upregulation, thereby mediating the production of class-switched and hypermutated autoantibodies in lupus B cells [23,34]. We have also shown that HoxC4 upregulation in lupus is further enhanced by estrogen [9] and, as suggested by our current findings, can be increased by APRIL. Together with researching showing delay of disease development in a murine model of lupus by selective antibody-mediated APRIL blockade [35], our data suggest significantly different roles of APRIL and BAFF for mediating the upregulation of AID and the induction of antibody and autoantibody responses.
Conclusions
In conclusion, our findings demonstrate that APRIL induces HoxC4 expression through NF-κB-mediated HoxC4 gene transcription, and HoxC4 in turn can induce AID expression by B cells. HoxC4 and AID are upregulated in lupus B cells and can mediate the production of class-switched and hypermutated autoantibodies by the B cells. On the other hand, excess level of APRIL correlate with activity of autoimmune diseases and titers of pathogenic autoantibodies caused by B cell abnormality. Consequently, our observations will serve basis to regulate the production of pathogenic autoantibodies in autoimmune patients.
Acknowledgements
This work was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (MEST) (No. 2009-0066904 to S.-R.P.). The research was also partially supported by NIH grant AI 079705 to P.C.
Abbreviations
- AID
activation-induced cytidine deaminase
- AP-1
activator protein-1
- APRIL
a proliferation-inducing ligand
- BAFF
B cell-activating factor belonging to the TNF family
- ChIP
Chromatin immunoprecipitation
- CREB
cAMP response element binding protein
- CSR
class switch recombination
- DN
dominant negative
- GC
germinal center
- Ig
immunoglobulin
- IL
interleukin
- LPS
lipopolysaccharide
- NF-κB
nuclear factor-κB
- NBE
NF-κB-binding element
- SHM
somatic hypermutation
- STAT
signal transducer and activator of transcription
References
- [1].Nagaoka H, Tran TH, Kobayashi M, Aida M, Honjo T. Preventing AID, a physiological mutator, from deleterious activation: regulation of the genomic instability that is associated with antibody diversity. Int Immunol. 2010;22:227–35. doi: 10.1093/intimm/dxq023. [DOI] [PubMed] [Google Scholar]
- [2].Xu Z, Zan H, Pone EJ, Mai T, Casali P. Immunoglobulin class-switch DNA recombination: induction, targeting and beyond. Nat Rev Immunol. 2012;12:517–31. doi: 10.1038/nri3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kim RJ, Kim HA, Park JB, Park SR, Jeon SH, Seo GY, Seo DW, Seo SR, Chun GT, Kim NS, Yie SW, Byeon WH, Kim PH. IL-4-induced AID expression and its relevance to IgA class switch recombination. Biochem Biophys Res Commun. 2007;361:398–403. doi: 10.1016/j.bbrc.2007.07.022. [DOI] [PubMed] [Google Scholar]
- [4].Sayegh CE, Quong MW, Agata Y, Murre C. E-proteins directly regulate expression of activation-induced deaminase in mature B cells. Nat Immunol. 2003;4:586–93. doi: 10.1038/ni923. [DOI] [PubMed] [Google Scholar]
- [5].Gonda H, Sugai M, Nambu Y, Katakai T, Agata Y, Mori KJ, Yokota Y, Shimizu A. The balance between Pax5 and Id2 activities is the key to AID gene expression. J Exp Med. 2003;198:1427–37. doi: 10.1084/jem.20030802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Lee CH, Melchers M, Wang H, Torrey TA, Slota R, Qi CF, Kim JY, Lugar P, Kong HJ, Farrington L, van der Zouwen B, Zhou JX, Lougaris V, Lipsky PE, Grammer AC, Morse HC., 3rd Regulation of the germinal center gene program by interferon (IFN) regulatory factor 8/IFN consensus sequence-binding protein. J Exp Med. 2006;203:63–72. doi: 10.1084/jem.20051450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Yadav A, Olaru A, Saltis M, Setren A, Cerny J, Livak F. Identification of a ubiquitously active promoter of the murine activation-induced cytidine deaminase (AICDA) gene. Mol Immunol. 2006;43:529–41. doi: 10.1016/j.molimm.2005.05.007. [DOI] [PubMed] [Google Scholar]
- [8].Xu Z, Pone EJ, Al-Qahtani A, Park SR, Zan H, Casali P. Regulation of aicda expression and AID activity: relevance to somatic hypermutation and class switch DNA recombination. Crit Rev Immunol. 2007;27:367–97. doi: 10.1615/critrevimmunol.v27.i4.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Mai T, Zan H, Zhang J, Hawkins JS, Xu Z, Casali P. Estrogen receptors bind to and activate the HOXC4/HoxC4 promoter to potentiate HoxC4-mediated activation-induced cytosine deaminase induction, immunoglobulin class switch DNA recombination, and somatic hypermutation. J Biol Chem. 2010;285:37797–810. doi: 10.1074/jbc.M110.169086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Pone EJ, Zhang J, Mai T, White CA, Li G, Sakakura JK, Patel PJ, Al-Qahtani A, Zan H, Xu Z, Casali P. BCR-signalling synergizes with TLR-signalling for induction of AID and immunoglobulin class-switching through the non-canonical NF-kappaB pathway. Nat Commun. 2012;3:767. doi: 10.1038/ncomms1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Park SR, Zan H, Pal Z, Zhang J, Al-Qahtani A, Pone EJ, Xu Z, Mai T, Casali P. HoxC4 binds to the promoter of the cytidine deaminase AID gene to induce AID expression, class-switch DNA recombination and somatic hypermutation. Nat Immunol. 2009;10:540–50. doi: 10.1038/ni.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Schaffer A, Kim EC, Wu X, Zan H, Testoni L, Salamon S, Cerutti A, Casali P. Selective inhibition of class switching to IgG and IgE by recruitment of the HoxC4 and Oct-1 homeodomain proteins and Ku70/Ku86 to newly identified ATTT cis-elements. J Biol Chem. 2003;278:23141–50. doi: 10.1074/jbc.M212952200. [DOI] [PubMed] [Google Scholar]
- [13].Kim EC, Edmonston CR, Wu X, Schaffer A, Casali P. The HoxC4 homeodomain protein mediates activation of the immunoglobulin heavy chain 3' hs1,2 enhancer in human B cells. Relevance to class switch DNA recombination. J Biol Chem. 2004;279:42258–69. doi: 10.1074/jbc.M407496200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Litinskiy MB, Nardelli B, Hilbert DM, He B, Schaffer A, Casali P, Cerutti A. DCs induce CD40-independent immunoglobulin class switching through BLyS and APRIL. Nat Immunol. 2002;3:822–9. doi: 10.1038/ni829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Castigli E, Wilson SA, Scott S, Dedeoglu F, Xu S, Lam KP, Bram RJ, Jabara H, Geha RS. TACI and BAFF-R mediate isotype switching in B cells. J Exp Med. 2005;201:35–9. doi: 10.1084/jem.20032000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kim HA, Seo GY, Kim PH. Macrophage-derived BAFF induces AID expression through the p38MAPK/CREB and JNK/AP-1 pathways. J Leukoc Biol. 2011;89:393–8. doi: 10.1189/jlb.1209787. [DOI] [PubMed] [Google Scholar]
- [17].Nakamura M, Kondo S, Sugai M, Nazarea M, Imamura S, Honjo T. High frequency class switching of an IgM+ B lymphoma clone CH12F3 to IgA+ cells. Int Immunol. 1996;8:193–201. doi: 10.1093/intimm/8.2.193. [DOI] [PubMed] [Google Scholar]
- [18].Murray PD, McKenzie DT, Swain SL, Kagnoff MF. Interleukin 5 and interleukin 4 produced by Peyer's patch T cells selectively enhance immunoglobulin A expression. J Immunol. 1987;139:2669–74. [PubMed] [Google Scholar]
- [19].Lin SC, Stavnezer J. Activation of NF-kappaB/Rel by CD40 engagement induces the mouse germ line immunoglobulin Cgamma1 promoter. Mol Cell Biol. 1996;16:4591–603. doi: 10.1128/mcb.16.9.4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lin SC, Wortis HH, Stavnezer J. The ability of CD40L, but not lipopolysaccharide, to initiate immunoglobulin switching to immunoglobulin G1 is explained by differential induction of NF-kappaB/Rel proteins. Mol Cell Biol. 1998;18:5523–32. doi: 10.1128/mcb.18.9.5523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Arsura M, Panta GR, Bilyeu JD, Cavin LG, Sovak MA, Oliver AA, Factor V, Heuchel R, Mercurio F, Thorgeirsson SS, Sonenshein GE. Transient activation of NF-kappaB through a TAK1/IKK kinase pathway by TGF-beta1 inhibits AP-1/SMAD signaling and apoptosis: implications in liver tumor formation. Oncogene. 2003;22:412–25. doi: 10.1038/sj.onc.1206132. [DOI] [PubMed] [Google Scholar]
- [22].Park SR, Lee JH, Kim PH. Smad3 and Smad4 mediate transforming growth factor-beta1-induced IgA expression in murine B lymphocytes. Eur J Immunol. 2001;31:1706–15. doi: 10.1002/1521-4141(200106)31:6<1706::aid-immu1706>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- [23].White CA, Seth Hawkins J, Pone EJ, Yu ES, Al-Qahtani A, Mai T, Zan H, Casali P. AID dysregulation in lupus-prone MRL/Fas(lpr/lpr) mice increases class switch DNA recombination and promotes interchromosomal c-Myc/IgH loci translocations: modulation by HoxC4. Autoimmunity. 2011;44:585–98. doi: 10.3109/08916934.2011.577128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Bossen C, Schneider P. BAFF, APRIL and their receptors: structure, function and signaling. Semin Immunol. 2006;18:263–75. doi: 10.1016/j.smim.2006.04.006. [DOI] [PubMed] [Google Scholar]
- [25].Hatzoglou A, Roussel J, Bourgeade MF, Rogier E, Madry C, Inoue J, Devergne O, Tsapis A. TNF receptor family member BCMA (B cell maturation) associates with TNF receptor-associated factor (TRAF) 1, TRAF2, and TRAF3 and activates NF-kappa B, elk-1, c-Jun N-terminal kinase, and p38 mitogen-activated protein kinase. J Immunol. 2000;165:1322–30. doi: 10.4049/jimmunol.165.3.1322. [DOI] [PubMed] [Google Scholar]
- [26].Sen CK, Packer L. Antioxidant and redox regulation of gene transcription. FASEB J. 1996;10:709–20. doi: 10.1096/fasebj.10.7.8635688. [DOI] [PubMed] [Google Scholar]
- [27].Flohé L, Brigelius-Flohé R, Saliou C, Traber MG, Packer L. Redox regulation of NF-kappa B activation. Free Radic Biol Med. 1997;22:1115–26. doi: 10.1016/s0891-5849(96)00501-1. [DOI] [PubMed] [Google Scholar]
- [28].Yu G, Boone T, Delaney J, Hawkins N, Kelley M, Ramakrishnan M, McCabe S, Qiu WR, Kornuc M, Xia XZ, Guo J, Stolina M, Boyle WJ, Sarosi I, Hsu H, Senaldi G, Theill LE. APRIL and TALL-I and receptors BCMA and TACI: system for regulating humoral immunity. Nat Immunol. 2000;1:252–6. doi: 10.1038/79802. [DOI] [PubMed] [Google Scholar]
- [29].Mackay F, Silveira PA, Brink R. B cells and the BAFF/APRIL axis: fast-forward on autoimmunity and signaling. Curr Opin Immunol. 2007;19:327–36. doi: 10.1016/j.coi.2007.04.008. [DOI] [PubMed] [Google Scholar]
- [30].Schneider P. The role of APRIL and BAFF in lymphocyte activation. Curr Opin Immunol. 2005;17:282–9. doi: 10.1016/j.coi.2005.04.005. [DOI] [PubMed] [Google Scholar]
- [31].Castigli E, Scott S, Dedeoglu F, Bryce P, Jabara H, Bhan AK, Mizoguchi E, Geha RS. Impaired IgA class switching in APRIL-deficient mice. Proc Natl Acad Sci U S A. 2004;101:3903–8. doi: 10.1073/pnas.0307348101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Hendriks J, Planelles L, de Jong-Odding J, Hardenberg G, Pals ST, Hahne M, Spaargaren M, Medema JP. Heparan sulfate proteoglycan binding promotes APRIL-induced tumor cell proliferation. Cell Death Differ. 2005;12:637–48. doi: 10.1038/sj.cdd.4401647. [DOI] [PubMed] [Google Scholar]
- [33].Ingold K, Zumsteg A, Tardivel A, Huard B, Steiner QG, Cachero TG, Qiang F, Gorelik L, Kalled SL, Acha-Orbea H, Rennert PD, Tschopp J, Schneider P. Identification of proteoglycans as the APRIL-specific binding partners. J Exp Med. 2005;201:1375–83. doi: 10.1084/jem.20042309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Zan H, Zhang J, Ardeshna S, Xu Z, Park SR, Casali P. Lupus-prone MRL/faslpr/lpr mice display increased AID expression and extensive DNA lesions, comprising deletions and insertions, in the immunoglobulin locus: concurrent upregulation of somatic hypermutation and class switch DNA recombination. Autoimmunity. 2009;42:89–103. doi: 10.1080/08916930802629554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Huard B, Tran NL, Benkhoucha M, Manzin-Lorenzi C, Santiago-Raber ML. Selective APRIL blockade delays systemic lupus erythematosus in mouse. PLoS One. 2012;7:e31837. doi: 10.1371/journal.pone.0031837. [DOI] [PMC free article] [PubMed] [Google Scholar]