Table1.
Chiral Catalyst Screena
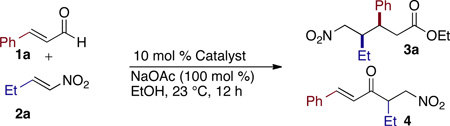 | |||||
|---|---|---|---|---|---|
| entry | Catalyst | 3a:4 | Yield 3a (%)b | dr (syn/anti)c | ee (%)d |
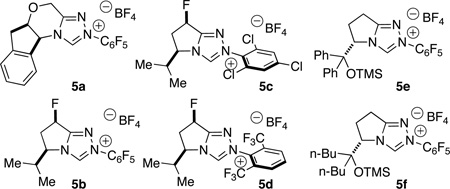
| 1 | 5a | 2:1 | 25 | 17:1 | −40 |
| 2 | 5b | 1:1 | 42 | 17:1 | −83 |
| 3 | 5c | 5:1 | 70 | 2:1 | −22 |
| 4 | 5d | 6:1 | 60 | 15:1 | −30 |
| 5 | 5e | >20:1 | 16 | 17:1 | 95 |
| 6 | 5f | >20:1 | 49 | 17:1 | 93 |
Reactions were conducted with 1.5 equiv of 1 and 1.0 equiv of 2.
Isolated yield after chromatography.
Diastereoselectivity determined by 1H NMR of the unpurified reaction mixture.
Enantiomeric excess was determined by HPLC analysis on a chiral stationary phase.
