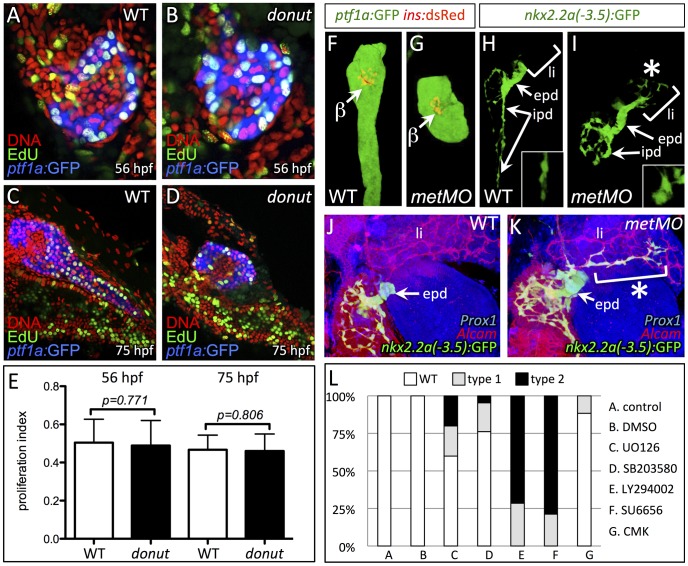
Figure 4. Cell migration, but not proliferation, is dysregulated in donuts908 mutants.
(A–D) EdU incorporation assay in WT (A,C) and donuts908 mutant (B,D) animals at 56 (A,B) and 75 (C,D) hpf. (E) Quantification of proliferation assay data shows no significant change in acinar cell proliferation at early or late stages of pancreatic tail formation. (F–I) Morphology of exocrine tissues at 84 hpf in WT (F,H) and metMO-injected (G,I) larvae revealed by Tg(ptf1a:GFP) (acinar; F,G) or Tg(nkx2.2a(-3.5 kb):GFP) (duct; H,I) expression. In metMO-injected embryos, acinar and ductal cells fail to migrate caudally, and remain near the principal islet (β). In metMO larvae, ductal cells exhibit a more rounded morphology in the exocrine pancreas (insets) and are also observed in the liver region (asterisk). (J,K) 84 hpf WT (J) and metMO-injected (K) Tg(duct:GFP) larvae stained with Prox1 (blue) and Alcam (red). Pancreatic ductal cells are ectopically localized in the liver (li) along tracts of biliary ducts. (L) Quantification of pancreatic tail defects in small molecule treated larvae; inhibitors of the MAPK pathway (UO126, SB203580) and furin function (CMK) had little effect on pancreatic tail outgrowth, while inhibitors of PI3K (LY294002) and STAT3 (SU6656) function mimicked the type 1 and type 2 donut phenotypes. epd, extrapancreatic duct.