Figure 3.
Mutations in mtv1 and nev/agd5 Specifically Affect Vacuolar Transport of Endogenous Proteins and Do Not Alter Endocytosis, Polar Targeting, Endosomal Recycling, or Secretion.
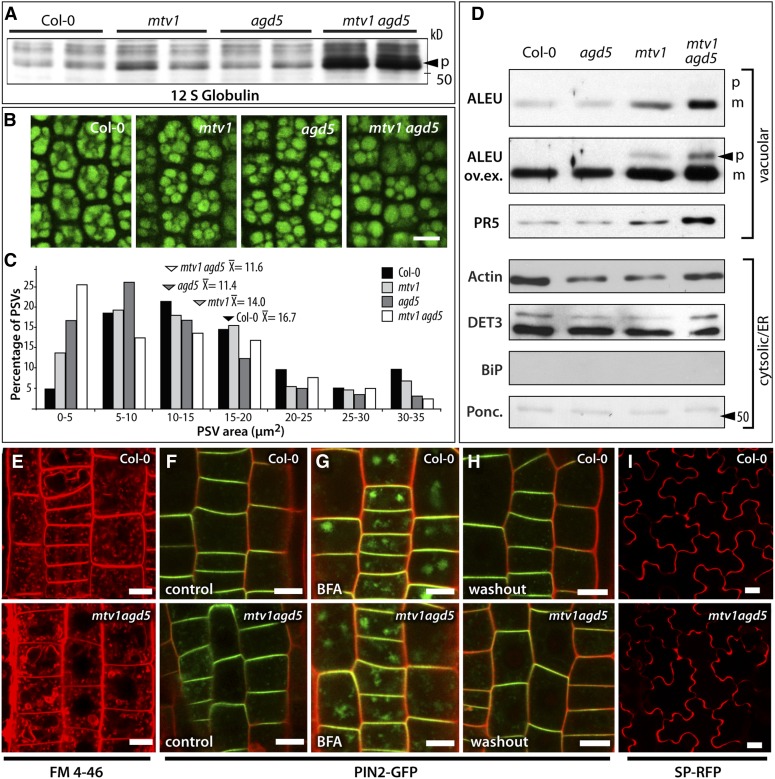
(A) Immunoblot analysis of seed protein extracts with 12S globulin antibody; arrowhead indicates the 52-kD precursor (p).
(B) Autofluorescence of protein storage vacuoles (PSVs) in hypocotyls of mature embryos from dry seeds.
(C) Size distribution of PSVs from each genotype is displayed in a bar graph (number of PSVs measured: n = 470 for Col-0, n = 566 for mtv1, n = 661 for agd5, and n = 660 for mtv1agd5). The mean PSV size (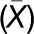 ) of the different genotypes is shown above the arrowheads. The PSVs were significantly smaller in mtv1 (
) of the different genotypes is shown above the arrowheads. The PSVs were significantly smaller in mtv1 (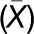 = 14.0 μm2, P = 4.4 × 10−5, unpaired t test), agd5 (
= 14.0 μm2, P = 4.4 × 10−5, unpaired t test), agd5 (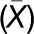 = 11.4 μm2, P = 1.4 × 10−16, unpaired t test), and mtv1agd5 (
= 11.4 μm2, P = 1.4 × 10−16, unpaired t test), and mtv1agd5 (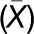 = 11.6 μm2, P = 1.6 × 10−15, unpaired t test) than in wild-type embryos (
= 11.6 μm2, P = 1.6 × 10−15, unpaired t test) than in wild-type embryos (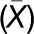 = 16.7 μm2).
= 16.7 μm2).
(D) Immunoblot analysis of extracellular fluid from fully expanded leaves of wild-type, single, and double mutants. ALEU, Aleurain proteinase; ALEU ov.ex., same as above but with increased exposure time to reveal an unprocessed precursor band (p, precursor form; m, mature form); PR5, thaumatin-like protein; DET3, subunit C of the V-ATPase; BiP, luminal binding protein BiP; Ponceau loading control.
(E) FM4-64 as marker for endocytotic uptake. The double mutant shows similar levels of intracellular (i.e., endocytosed) signal.
(F) to (H) PIN2-GFP signal (green) in propidium iodide (red) counterstained root epidermal cells.
(F) PIN2-GFP localizes to the apical polar domain of the wild type and the double mutant.
(G) PIN2-GFP is incorporated into BFA bodies after 1 h of BFA treatment in the wild type and double mutant.
(H) PIN2-GFP relocates to the apical plasma membrane 2 h after BFA was washed out in the wild type and the mutant.
(I) The secretory marker SP-RFP is secreted to the extracellular space of cotyledon epidermal cells in the wild type and the double mutant. Bars = 10 µm.