Figure 10.
AMSH1 and ESCRT-III Are Important for Autophagy and Autophagy-Mediated Physiological Responses in Plants.
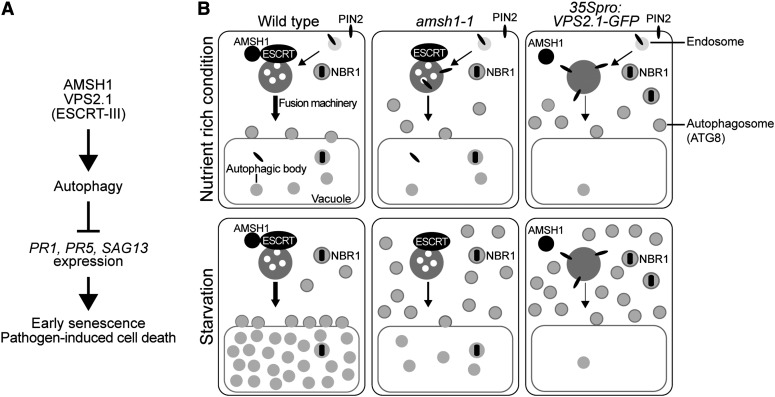
(A) AMSH1 and VPS2.1 functions are important for autophagic degradation. As previously reported, functional autophagy represses PR and SAG gene expression, induction of which causes early senescence and pathogen-induced cell death.
(B) amsh1-1 and 35Spro:VPS2.1-GFP are impaired in the degradation of autophagosomes to different extents. In wild-type cells, plasma membrane–localized PIN2 is endocytosed via the MVB pathway. The selective autophagy cargo receptor and substrate NBR1 is delivered to the vacuole via autophagosomes. Autophagosomes fuse with the vacuole in order to degrade its contents. Factors required for the recognition and fusion of autophagosomes with the vacuolar membrane as well as proteases responsible for the degradation of autophagic bodies may be transported via an MVB-dependent pathway. Upon carbon deprivation in the dark, autophagic recycling is highly upregulated. The weak amsh1-1 knockdown mutant is still capable of endocytic and autophagic degradation under optimal growth condition. However, when bulk autophagy is highly activated upon dark-induced carbon starvation, amsh1-1 accumulates ATG8 and shows less autophagic bodies in the vacuole, indicative for impaired autophagic degradation. When ESCRT-III function is disturbed by overexpressing VPS2.1-GFP, both endocytosis and autophagic recycling is strongly inhibited even under optimal growth conditions, leading to the accumulation of ATG8, NBR1, and PIN2. Accumulation of ATG8 increases when bulk autophagy is activated in the dark. Taken together, intact AMSH1 and ESCRT-III (VPS2.1) are essential for proper autophagic degradation.