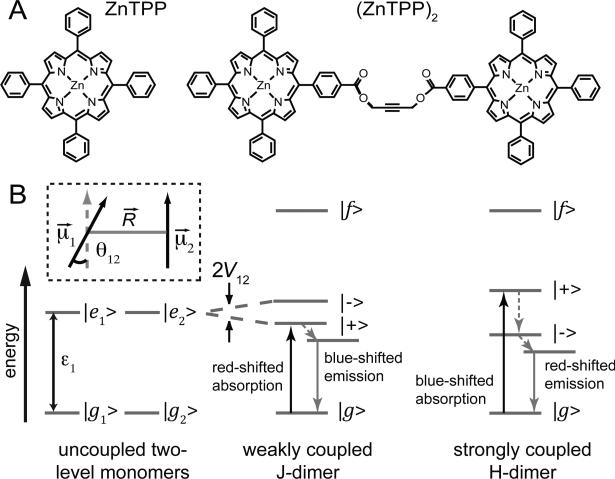
Figure 1.
(A) Structures of the chromophores used in this study, zinc tetraphenylporphyrin (ZnTPP) and a covalently linked dimer of ZnTPP (ZnTPP)2. (B) Energy level diagrams of two degenerate two-level molecules. Electronic coupling results in a four-level dimer with a single ground state |g〉, two non-degenerate singly-excited states |±〉, and a doubly-excited state |f〉. For a ‘weakly coupled J-dimer’ (i.e., with a head-to-tail dipole arrangement), the |+〉 state is shifted to lower energy. The situation is juxtaposed for a ‘strongly coupled H-dimer’ (i.e., a side-by-side dipole arrangement). Because transitions involving the |+〉 state are favored, absorption is red-shifted for a J-dimer, while it is blue-shifted for an H-dimer. After excited state relaxation, emission occurs from the lowest-energy singly-excited state, which is lower in energy for the H-dimer than the J-dimer due to the H-dimer's stronger coupling. Therefore, fluorescence from the H-dimer occurs on the red edge of the emission line shape while emission from the J-dimer occurs on the blue edge of the emission line shape.