My teacher, guide, and my mentor Prof. K. S. Mani aptly called “Father of Indian Epileptology” has first described hot water epilepsy way back in late 70’s. Some people even doubted whether such an entity exists. As I used to see these cases almost in the every outpatient clinic at National Institute of Mental health and neurosciences, I started thinking about it and many questions arise in my mind. This formed my research questions and started working on since 1980’s. Today, I am giving my talk on the culminated research experience, which I have acquired in the last three decade. I have titled my talk as “Bridge over Troubled Waters,” which is very famous poem. I have taken the literal meaning here and look at the water which posed the problem in the form of Epilepsy” How we have gone on to solve this mystery?
Introduction
Seizures that precipitated by a sensory stimulus are described as “reflex or sensory epilepsy” are interesting, and often provide insight into the pathogenesis of epilepsy in general and reflex epilepsy in particular. Penfield was the first author to use the term “sensory precipitation of seizures” in 1941 as the seizure-inducing mechanism rather than the cause.[1] One of such epilepsy is precipitated by the stimulus of bathing with hot water pouring over the head and better known as hot water epilepsy (HWE).[2–8] It is also variably known as water-immersion epilepsy or bathing epilepsy.[9–12]
History
Though, it was first described way back in 1945 from New Zealand,[13] there were isolated reports from all round the world: Australia,[14] United States of America,[15] Canada,[5] United Kingdom,[16] and Japan.[17,18] However, a large number of patients with this type of HWE have been reported from India, particularly south India.[6–9] A large cohort of 279 cases of HWE from a University Hospital and Tertiary Care Centre, National Institute of Mental health and Neurosciences (NIMHANS), was evaluated over a 4 year period (1980-1983).[6,7] The detailed initial description and subsequent works on this geographically specific epilepsy syndrome - HWE have been carried out by two researchers K. S. Mani and P. Satishchandra, both belonging to the same region and the institute.[2,3,6–19,20]
Epidemiology
A House-to-house Bangalore urban-rural neuroepidemiologic survey of 102,557 population from South India, reported that HWE accounts for 6.9% of all epilepsies in this community, giving a prevalence rate of 60 per 100,000 (unpublished data). Mani et al., published an epidemiologic study from Yelandur, a rural area near Mysore from Karnataka, and quoted a prevalence rate of 255/100,000 for HWE.[20] The classification proposed by the International League Against Epilepsy task force in 2001 includes HWE under the reflex epilepsies.[21] In a recent study from this center on 70 patients, majority of the patients belonged to Mandya: 30.5%, Ramanagara: 30.0% and Mysore: 15.2% districts of Karnataka.[22]
Phenotypic description
Like any other South Indians take bath daily, but as a customary practice of washing of the head is done generally once in 2-15 days. Temperature of the hot water used for bathing ranges between 40°C and 50°C (ambient room temperature, 25-30°C). Usually water is collected in a bucket, and water is poured over the body or head by using a tumbler or mug in this part of the country. Following that episodes of HWE are precipitated in some individuals. However, less commonly HWE in people using a shower or tub bathing have also been noted. This type of HWE has been reported as isolated cases from people having hot showers or tub baths from all over the world, including the United States, Canada, UK, Ireland, and Australia. Small series of HWE have been published from Japan and recently from Turkey.[23]
In a recent study on 70 patients of HWE from this center by Meghana et al., (2012) 45 subjects (M:F 37:8; age at presentation: 24.6 ± 10.5 years; age at the onset of HWE 18.7 ± 10.2 years) had features of ‘HWE alone’ while 25 subjects (M:F = 18:7; age at presentation: 26.8 ± 7.9 years) had ‘HWE with spontaneous seizure’ (age at the onset of HWE in “HWE with spontaneous seizure’: 16.8 ± 10.3 years; duration of ‘HWE with spontaneous seizure”: 116.5 ± 66.9 months; duration of “spontaneous seizure”: 91.2 ± 8.9 months; average interval between “HWE and spontaneous seizure”: 33.9 ± 0.6 months). Table 1 depicts the phenotypic and demographic features of the two subgroups of patients with HWE in the series by Meghna et al., (2012; 22). Among 45 cases of “HWE alone”, 10 used had seizures while pouring even luke warm water and one patient had a seizure while washing face with hot water. 23 subjects out of 70 had 1:1 episodes. Among those with HWE alone, eight subjects could not avoid hot water bath and had increased frequency of attacks (2-3 seizures/week). One-third of patients with ‘HWE alone’ subgroup, the attacks occurred whenever they used to take hot water head bath (1:1): Men - 35% and women - 17%. In those with “HWE with spontaneous seizure” subgroup, 10 patients had 2-3 seizures/week. Ten patients had 1:1 episode of seizures with hot water bath. In the “HWE with spontaneous seizure” subgroup, the manifestation interval between reflex HWE and spontaneous seizure was variable: <2 weeks: 8; <6 months: 4; <2 years: 13. Children who got “HWE alone” in early age (age of onset was ≤ 12 years) progressed to develop “spontaneous seizure”. In the group of patients of HWE with spontaneous seizure (n = 25), 14 subjects (56%) had onset of HWE on or before 12 years of age. The family history of spontaneous epilepsy was 33.3% in “HWE alone” and 36.4% in “HWE with spontaneous seizure.” Family history of HWE in “HWE alone” group was 26.8% and 13.6% in “HWE with spontaneous seizure.” About, 22% of patients with “HWE alone” and 18.2% of those with “HWE with spontaneous seizure” had a previous history of febrile convulsion.
Table 1.
Comparison of various clinical features of both the subgroups of HWE
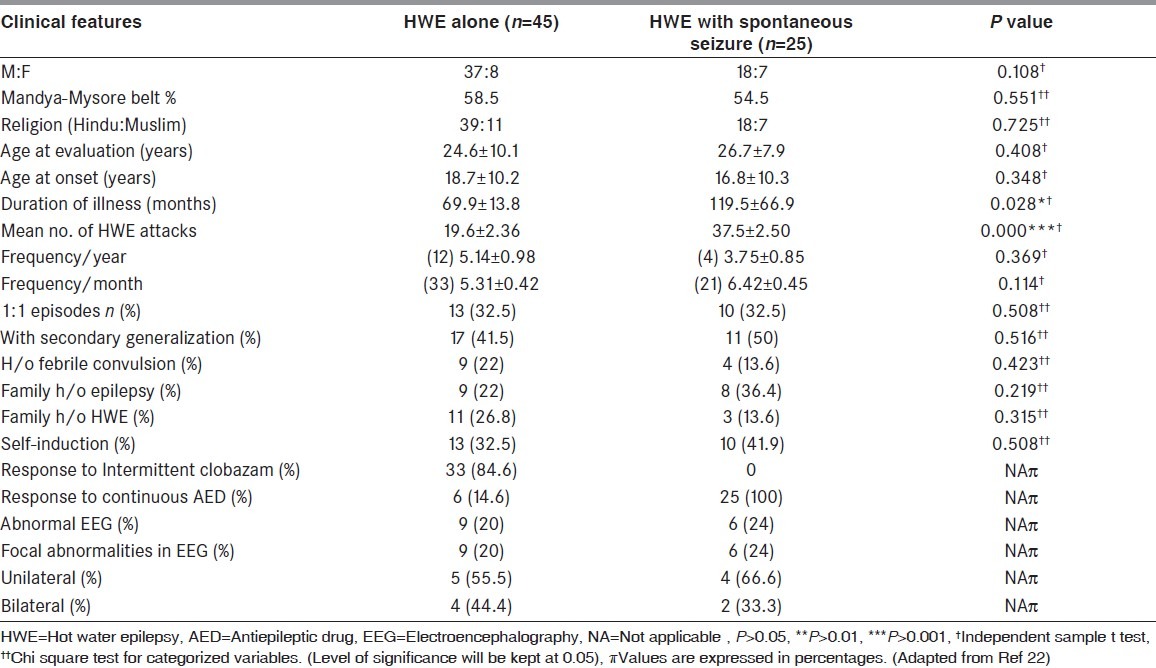
Children are more frequently affected, although, it has been reported in adults from our center.[2,3,6–8] Males are affected more frequently than female subjects (2-2.5:1). Generally, the frequency of these seizures depends on the frequency of head bathing. Five to 10% of these patients have seizures even during a body bath when water is not poured over the head, at a later stage in the natural history. The most common type of seizures is of complex partial with or without secondary generalization. It is characterized by a dazed look, sense of fear, irrelevant speech, and visual and auditory hallucinations with complex automatisms. About 1/3rd of all reported cases have primary generalized tonic-clonic seizures. These seizures have been witnessed in the laboratory and have been documented on a video in few instances.[5–7,15,17,22] About 10% of these patients, who express intense desire and continue to pour hot water over the head until they lose consciousness, could be considered to have “self-induced HWE” (unpublished observation). These seizures last 30 s to 3 min and could manifests either at the beginning or at the end of the bath. Spontaneous non-reflex epilepsy has been reported to occur few years later in 16-38% of patients.[3,6–8,22] A history of epilepsy among the family members has been reported in 7-22.6% of cases. There are no neurologic deficits in these patients.
Seizure semiology was ascertained in the study by Meghana et al.,[22] was based on history, i.e., eye witness account (n = 62) or video documentation (n = 8). Subjects with “HWE alone” had complex partial seizure (CPS) in 58.5% and CPS with secondary generalization in 41.5%. Voluntary act of taking bath stopped when seizure began, and similarly, when the relatives were pouring hot water on the head, they could recognize the onset and stopped further pouring of hot water. Majority of the patients (60%) got an attack wherein they would sit flexing their body and closing ears with both hands with aura of fear, for few seconds followed by the seizure. They had varied manifestations which included loss of orientation and awareness, staring look, behavioral arrest, irrelevant speech, déjà vu, among feeling like fainting etc., each lasting for few seconds to minutes. By and large, except for a tendency to look around aimlessly, rarely wandering (one), praying (one) there was no other motor phenomena. Post ictal headache and sleep were present. Eleven patients gave a history of secondary generalization at the end of the hot water head bath. Twenty five subjects of the “HWE with spontaneous seizure” subgroup had CPS during sleep with semiology of screaming loudly, tonic flexion of the body followed by secondary generalization. The phenomenon of “self-induction of HWE” was seen in 23 out of 70 patients (32.8%), who also had frequent attacks. They get an aura of pleasure during hot water both and would continue to pour the hot water on head until they had seizure. 18 patients also gave a history of self-limiting attacks, wherein patients could control the feeling of euphoria and thereby abort the attack. A comparison of clinical features of both the subgroups is provided in Table 1.
Electroencephalography features
Interictal scalp electroencephalography (EEG) is usually normal, but 15-20% might show diffuse abnormalities.[3–8] Lateralized or localized spike discharges in the anterior temporal regions have been reported in a few isolated cases.[15,21,22] Ictal EEG recording has its own technical limitations and is difficult to obtain; however, there are seven reports in the literature demonstrating the ictal EEG recordings during provocation in water-immersion epilepsy. They had demonstrated left temporal rhythmic delta activity,[15] sharp and slow waves in the left hemisphere bilateral spikes,[16] and temporal activity.[11,12] Simultaneous split-screen video-EEG recording in one patient with “bathing” epilepsy demonstrated delta waves starting from the right hemisphere with rapid secondary generalization.[24]
In the study by Meghana et al., (2012), EEG was performed in all 70 patients with HWE and was normal in 55 and abnormal in 15. In the ‘HWE alone’ subgroup (n = 45), nine had abnormal EEG while in the among “HWE with spontaneous seizure” subgroup (n = 25), six had abnormal EEG. The abnormalities in the majority were located in the fronto-temporal region on either or both sides [Figure 1].[22]
Figure 1.
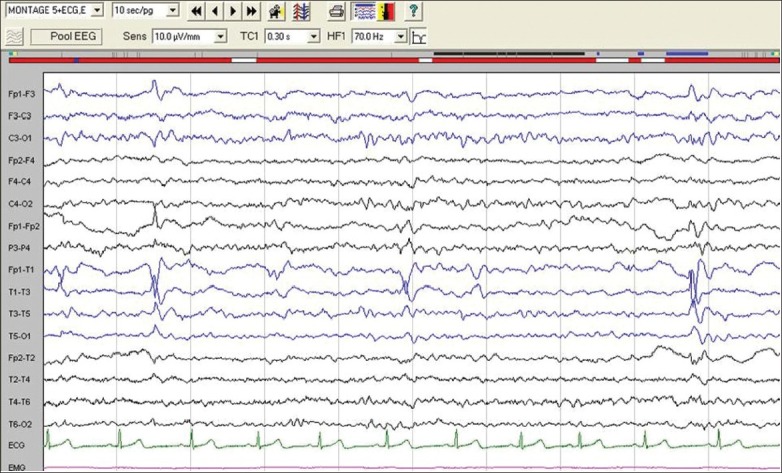
Electroencephalography of patient with hot water epilepsy with spontaneous seizures showing focal sharp wave discharges mainly from left temporal region
Pathogenesis
The exact pathogenesis of this unique form of reflex epilepsy is not known and therefore some hypotheses have been put forward. Stensman and Ursing[15] suggested that this type of epilepsy is precipitated by complex tactile and temperature dependent stimuli. Although, it was possible to provoke the seizure in the laboratory by pouring hot water over the heads of these patients, hot-water towels, sauna, or blowing hot air on the head failed to induce seizures, suggesting that the triggering stimulus is complex and would involve a combination of factors such as (a) contact of scalp with hot water, (b) temperature of water, and (c) specific cortical area of stimulation. As the CPSs are the commonest variety of seizure, and ictal EEG had demonstrated focal activity in the temporal or frontal lobe, Syzmonowicz and Meloff[5] suggested that there could be a structural lesion in the temporal lobe. However, computed tomography (CT) and magnetic resonance imaging (MRI) in patients with HWE have negated the presence of any focal structural lesions in the majority. Even if such lesion were present, it is still not clear whether the mechanism of seizure depends on locally increased neuronal excitability in the lesions, or pathologic involvement of lower centers such as hypothalamus, or both.[16] Shankar and Satishchandra reported pathological findings in three of their patients with HWE.[25] From 11% to 27% of the HWE patients reported from India had history suggestive of febrile convulsions before the development of this reflex epilepsy.[3,4,7,22] This association has not been noted from other parts of the world.
Repeated exposure of the heads of adult rats to hot water (45°C) induced experimental seizures, which is comparable to the phenomenon of kindling by repeated stimulation with subthreshold electrical current. Klauenberg and Sparber[26] called this “hyperthermic kindling.” Satishchandra et al.,[7,27] postulated that the similar phenomenon of “hyperthermic kindling” might be responsible for the development of HWE in humans. Further to understand the pathophysiological and pharmacological mechanisms underlying HWE, an experimental animal model mimicking HWE in its entirety ([a] precipitating stimulus, [b] the ictal events, and [c] EEG comparable to those of human HWE] was developed by the same group.[27,28] They demonstrated “hyperthermic kindling” in this model on repeated stimulation and confirmed it by Timm staining (OK). Translating this information to human, the authors recorded the body temperature through a thermistor kept inside the auditory canal in the susceptible human with HWE during a hot-water head bath.[29,20] This demonstrated a “rapid spurt” in the temperature of 2-3°F within a short span of 2 min. It takes 10-12 min for this temperature to return to the baseline, once the bath is completed. This is in comparison with the increase of 0.5-0.6°F noted in the normal healthy volunteers, which return to the baseline immediately, at the end of the bath. This suggests that this special form of induced hyperthermia could be responsible for causing HWE in these susceptible individuals.[28–30] We postulated that HWE patients probably have an aberrant thermoregulatory system and are extremely sensitive to the rapid increase in temperature occurring during hot water head baths, which precipitates seizures. This aberrant thermoregulation seems to be genetically determined, and further work to elucidate this hypothesis is under way. The rat model described simulates human HWE and gives new evidence that human HWE is a “hyperthermic” seizure with probable kindling process.[30–32]
Autonomic Involvement in HWE
We recently characterized the role of autonomic nervous system function in reflex epilepsy mainly in HWE. Comparison of the cardiac autonomic functions in patients with HWE (n = 90), spontaneous epilepsy (n = 40) and healthy volunteers (n = −51) was done. We evaluated the relationship between phenotypic observations and cardiac autonomic parameters in all the reflex epilepsies and non-reflex spontaneous epilepsy. This study has demonstrated an impaired sympatho-vagal balance characterized by increased sympathetic activity and reduced parasympathetic activity in patients with HWE. The HWE with spontaneous CPS had more deteriorating cardiac autonomic nervous system control than HWE due to long duration of illness. There was no significant effect of carbamazepine (CBZ) drug on ANS. In summary, reflex epilepsy have mild to moderate autonomic dysfunction. This study also supported the hypothesis of hypothalamic dysfunction and a distinctive sympathetic dysfunction in patients with HWE.[33,34]
Functional Imaging Observations
As there are no structural changes in the MRIs of this human HWE, it is more likely that functional changes in these susceptible human could induce seizures.
To demonstrate this, we recently conducted a study of interictal and ictal single-photon emission computed tomography (SPECT) scans in 10 HWE patients who had recurrent pure HWE, by using the ethylene cysteine dimer (ECD). All had MRI and interictal scalp EEG. Interictal 99 mTc SPECT scans were performed initially in all these patients by using a single-head scanner. After this, patients were stimulated with hot-water head bath after obtaining their written consent. Five (50%) patients who had HWE in the laboratory were administered intravenously 99 mTc ECD at the onset of the ictal event, and periictal SPECT scans were repeated. These were subtracted from corresponding interictal SPECT scans. This demonstrated ictal hypermetabolic uptake in the medial temporal structures and hypothalamus on the left in three and on the right in two patients, with spread to the opposite hemisphere. In a similar attempt to study, five patients with HWE underwent evaluation the SPECT - 3 ictal and 2 inter-ictal. There were changes in medial temporal region. This preliminary study clearly demonstrated functional involvement of these structures in triggering HWE.[35,36]
Genetics of HWE
Although positive family histories seen in about 10-23% HWE patients from Southern India, have suggested involvement of genetic etiology of the disorder,[3,6,7,22,37,38] toward identification of HWE genes were initiated. In an on-going effort to understand the molecular basis of HWE, we have examined seven HWE families with several of their members affected with the disorder, ascertained at NIMHANS. These studies have helped identification of two loci for HWE at chromosome 10q21.3-q22.3[39] and 4q24-q28.[40] Among the families analyzed, family 150 was the largest and comprised four generations with 10 affected and 20 apparently unaffected members [Figure 2]. This family was recruited through proband III: 10 who was diagnosed with HWE at the age of 15 years. His interictal EEG showed generalized discharges arising from the left temporal region of the brain. HWE in the family 150 and six additional families (number - 12, 14, 52, 227, 257, and 261) transmitted in an apparently autosomal dominant mode with incomplete penetrance. A whole genome-based linkage analysis involving about 400 microsatellite deoxyribonucleic acid (DNA) markers was carried out for family 150 and parametric two-point LOD scores were calculated considering autosomal dominant inheritance with 50-90% penetrance values, a disease allele frequency of 0.0001 and 1% phenocopy. Following whole-genome based analysis of family 150, the highest two-point LOD score obtained was 3.17 at recombination faction (θ) = 0 and penetrance value of 60%, for the marker D10S412 at chromosomal region 10q21 [Figure 2]. Analysis of five additional HWE families mentioned above, for markers on chromosome 10, further strengthened the evidence of linkage to the same chromosomal region with three out of five families providing support of linkage to the same sub-chromosomal region. The markers D10S201 and D10S581 defined the boundaries of the critical genetic interval. In a similar study of family 227 [Figure 2], significant linkage was detected on chromosome 4q24-q28 [Figure 2], with the highest two-point LOD score of 3.50 at recombination value (θ) of 0 for the marker D4S402. The markers D4S1572 and D4S2277 defined centromere-proximal and centromere-distal boundaries of this locus, respectively. The critical genetic interval spans about 24 mega bases of DNA. Another large family 300 of HWE with autosomal dominant inheritance pattern, significant linkage was detected on chromosome 9p24.3-p23 with the highest two-point LOD score of 3.42 at recombination value (θ) of 0 for the marker D9S286 [Figure 3].
Figure 2.
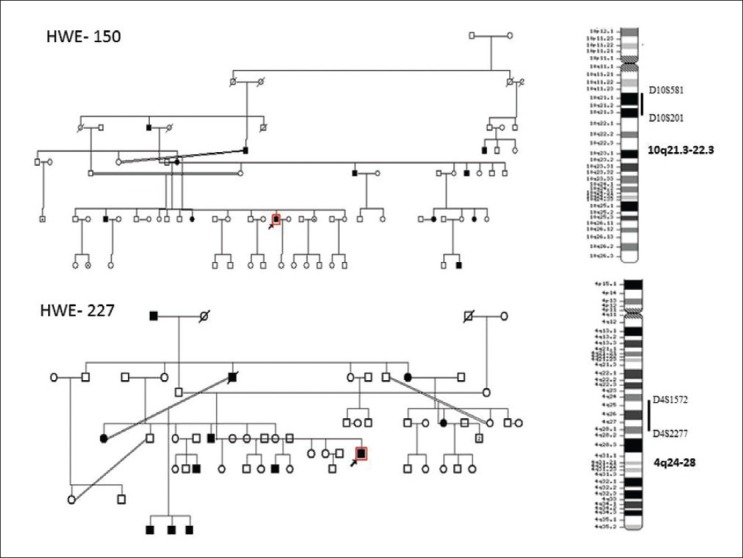
Upper half depicting the pedigree chart of the family 150 and the hot water epilepsy (HWE) loci at 10q21.2-q22.3. Lower half reveals the pedigree chart of family 227. Key recombinant events in III: 4, III: 5 and IV: 3 are indicated by arrows. Seizure types and age at onset are indicated beside symbols; The HWE linkage is depicted in the chromosome 4q24-q28
Figure 3.
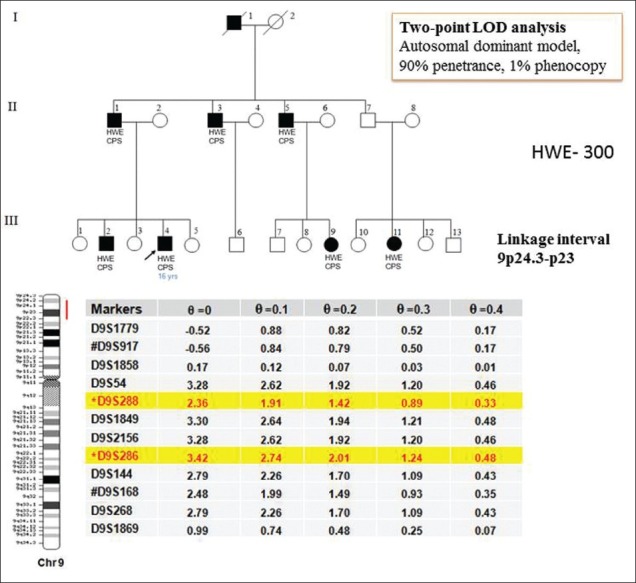
It shows the pedigree chart of the family 300 and the hot water epilepsy linkage is depicted in the chromosome 9p24.3-p23
Hot water bath triggering reflex epilepsy and high fever leading to febrile convulsions in children suggest apparently similar mechanisms. Genetic loci for familial febrile convulsions have been mapped to 2q23-q24, 3p24.2-p23, 5q14-q15, 5q31.1-q33.1, 6q22-q24, 8q13-q21, 18p11.2, 19p13 and 22q22.[41] However, the 10q21.3-q22.3 and 4q24-q28 loci identified for HWE [Figure 3] are different from the loci implicated in febrile convulsions suggesting that HWE and FC are independent clinical entities.
Reports of high prevalence of HWE in South India suggest role of a founder effect for the disorder. Alternatively, HWE may be genetically heterogeneous and gene-environment interaction could be responsible to explain increased prevalence of this in southern India.
Identification of two genetic loci for HWE is an important step towards isolation of the responsible genes. The 15 Mb critical interval at 10q21.3-q22.3 and 24 Mb critical interval at 4q24-q28 harbors several genes known to be expressed in the human brain. Among the genes known from the 10q21.3-q22.3 region, the ones coding for ion channel proteins, KCNMA1 and VDAC2, were the most obvious candidates for HWE as epilepsies caused by mutations in ion channels are a large subset of monogenic epilepsies. SLC 29A3 and SLC25A16, which are involved in regulation of neuronal ion flux also map in this region. However, sequence analysis of these four genes did not identify a potentially causative coding mutation making involvement of these genes somewhat unlikely. Similarly, two genes, SLC39A8 and TRPC3 at 4q24-q28 were examined for coding mutations but none were isolated. However, possibilities of variations in the gene copy number, variations in the transcript structures such as alternative spliced isoforms or promoter methylation at these genes need to be examined. Some insights about the genes, disturbances at which could cause temperature-dependent generation of seizures have come from animal models. Temperature-sensitive, seizure-like phenotypes are also known to occur in Drosophila carrying mutations in Shaker, a gene known to code for sodium channel proteins[42] Another Drosophila mutant, Nubian displays temperature-dependent seizures due to defect in phosphoglycerate kinase, an enzyme required for ATP generation in the terminal stage of the glycolytic pathway.[43] These findings suggest an involvement of diverse types of molecules in temperature-sensitive seizure phenotypes akin to HWE in human. Interestingly, genes involved in the nervous system development (NEUROG3 ), molecules involved in calcium signaling (CAMK 2G and MYOZ1), cell cycle and apoptosis regulator one, receptor of neuropeptide (TACR2 ), neuronal Ca(2+) -binding proteoglycans (SPOCK 2), and sphingolipid activator proteins, map in the 10q21.3-q22.3 region and are potential candidates to be examined. Genes for neurodevelopment (NEUROG2, ANK2), Calcium signaling (CAMK2D) and in the formation of myelin membranes (UGT 8) are potentially strong candidates for the disorder in the 4q24-q28 region. Further work in the analysis of these and other such genes to identify the gene responsible for HWE is in progress.
Management
HWE in human are often managed in two ways previously: (a) using lukewarm water for a head bath or sponging with hot towels,[2–8,22] and (b) use of conventional antiepileptic drugs (AEDs) such as phenytoin (PHT) or CBZ. Follow-up of 208 patients with HWE treated with conventional AEDs for a mean period of 14 ± 12.9 months (range, 6-60 months) showed that 60% could be easily controlled, 18.3% had a 50% reduction in frequency, but others continued to have seizures at their last follow-up.[7,22] It is interesting to note that 10% of these HWE patients exhibit compulsive behavior, that is, self-induced HWE. From 16% to 38% of subjects with HWE continue to have seizures even during regular baths and develop non-reflex seizures during follow-up [Table 1]. This is indirect evidence for the phenomenon of “hyperthermic kindling” in human, although the occurrence of kindling in human is still controversial. In view of the observation that HWE is a type of “hyperthermic” seizure akin to febrile convulsions, Satishchandra et al.,[32] evolved a newer method of intermittent oral prophylaxis with benzodiazepines. They advocated the use of 5-10 mg of oral clobazam (CLB) 1.5-2 h before a head bath and avoid it every day. These patients do not require any other AEDs on a regular basis, thereby minimizing the cost and side effects of these AEDs. Conventional AEDs are to be used only if the patients develop non-reflex seizures apart from HWE.[34] Analysis of 304 patients with HWE was carried out: (a) pure HWE (n = 198; 65.1%); (b) HWE plus (n = 62; 20.4%); (c) defaulters (n = 44; 14.5%). Three fourths of patients on intermittent CLB (group a) showed excellent response and additional AEDs might be justified in the setting of poor therapeutic response and/or coexistent non-reflex epilepsy.[6,7]
In the recent report by Meghana et al., (2012) from this center, it was found that patients with “HWE alone” (n = 45) need to avoid hot water head bath and continuous AEDs prophylaxis is required in HWE with spontaneous seizures. At initial evaluation, majority of the “HWE alone” subjects (68%) were drug naive. All the patients were advised to avoid hot water bath. Intermittent CLB (5/10 mg) was advised who could not avoid having hot water head bath on some occasions. They were advised tablet CLB (5/10 mg) about 60-90 minutes before hot water head bath. After 3-8 months (mean: 5.5 ± 0.74 months) of follow-up, only 6/45 individuals stopped taking hot water head bath and were seizure free. The remaining 39 patients were on intermittent CLB. Thirteen patients took CLB 1-2/week while six patients required even 3-4/week. Thirty three patients (84.6%) were seizure free with intermittent tablet CLB but remaining six patients had uncontrolled seizures. They were prescribed tablet CBZ (600 mg/day) and all were seizure free. At initial evaluation, 5 among 25 patients of the HWE with spontaneous seizure subgroup were drug naïve, 16 patients were on intermittent CLB and 4 were already on continuous AEDs (phenobarbitone [60 mg/day] and PHT [200 mg/day]-2 each). Drug naïve and intermittent tablet CLB patients (n = 21) were prescribed CBZ (800 mg/day) and were advised to use luke warm water for head bath. After a short-term follow-up of 3-8 months (mean: 5.5 ± 0.7 months), all were seizure free.[22]
Conclusions
The development of experimental animal models and genetic analysis of human HWE patients have given new insights into the pathogenesis of this unique type of epilepsy as a type of “hyperthermic” seizure. Aberrant thermoregulation in the genetically susceptible population with possible coexisting environmental influences could be the mechanism responsible for this type of epilepsy. Moreover, practice of using intermittent CLB as prophylaxis rather than continuous usage of conventional AEDs has been established as the specific mode of treatment for this type of epilepsy.
Acknowledgment
We thank Dr. G. R. Ullal, S. K. Shankar, K. G. Kallur, S. Dilip, G. Gadre, Dr. R. Rathnapriya, Dr. A. Anand, Dr. A. Meghana for their association with research work on HWE at various stages.
Footnotes
Source of Support: Nil
Conflict of Interest: Nil.
References
- 1.Gastaut H. Dictionary of epilepsy. Part I. Geneva: World Health Organization; 1973. [Google Scholar]
- 2.Mani KS, Gopalakrishnan PN, Vyas JN, Pillai MS. “Hot-water epilepsy” - A peculiar type of reflex epilepsy. A preliminary report. Neurol India. 1968;16:107–10. [PubMed] [Google Scholar]
- 3.Mani KS, Mani AJ, Ramesh CK. Hot-water epilepsy - A peculiar type of reflex epilepsy: Clinical and EEG features in 108 cases. Trans Am Neurol Assoc. 1974;99:224–6. [PubMed] [Google Scholar]
- 4.Subrahmanayam HS. Hotwater epilepsy. Neurology (India) 1972;20(Suppl 2):241–3. [Google Scholar]
- 5.Szymonowicz W, Meloff KL. Hot water epilepsy. Can J Neurol Sci. 1978;5:247–51. doi: 10.1017/s0317167100024616. [DOI] [PubMed] [Google Scholar]
- 6.Satishchandra P, Shivaramakrishna A, Kaliaperumal VG. Hot water epilepsy: Avariant of reflex epilepsy in parts of South India. J Neurol. 1985;232(Suppl):212. [Google Scholar]
- 7.Satishchandra P, Shivaramakrishana A, Kaliaperumal VG, Schoenberg BS. Hot-water epilepsy: A variant of reflex epilepsy in southern India. Epilepsia. 1988;29:52–6. doi: 10.1111/j.1528-1157.1988.tb05098.x. [DOI] [PubMed] [Google Scholar]
- 8.Satishchandra P. Hot-water epilepsy. Epilepsia. 2003;44(Suppl 1):29–32. doi: 10.1046/j.1528-1157.44.s.1.14.x. [DOI] [PubMed] [Google Scholar]
- 9.Velmurugendran CU. Reflex epilepsy. J Neurol. 1985;232(Suppl):212. [Google Scholar]
- 10.Mofenson HC, Weymuller CA, Greensher J. Epilepsy due to water immersion: an unusual case of reflex sensory epilepsy. JAMA. 1965;191:600–1. doi: 10.1001/jama.1965.03080070084019. [DOI] [PubMed] [Google Scholar]
- 11.Shaw NJ, Livingston JH, Minns RA, Clarke M. Epilepsy precipitated by bathing. Dev Med Child Neurol. 1988;30:108–11. doi: 10.1111/j.1469-8749.1988.tb04731.x. [DOI] [PubMed] [Google Scholar]
- 12.Lenoir P, Ramet J, De Meirleir L, D’Allest AM, Desprechins B, Loeb H. Bathing-induced seizures. Pediatr Neurol. 1989;5:124–5. doi: 10.1016/0887-8994(89)90040-4. [DOI] [PubMed] [Google Scholar]
- 13.Allen IM. Observation on cases of reflex epilepsy. N Z Med J. 1945;44:135–42. [Google Scholar]
- 14.Keipert JA. Epilepsy precipitated by bathing: Water-immersion epilepsy. Aust Paediatr J. 1969;5:244–7. [Google Scholar]
- 15.Stensman R, Ursing B. Epilepsy precipitated by hot water immersion. Neurology. 1971;21:559–62. doi: 10.1212/wnl.21.5.559. [DOI] [PubMed] [Google Scholar]
- 16.Parsonage MJ, Moran JH, Exley KA. Epileptology Proceedings of the 7th Internatational Symposium on Epilepsy. Stuttgart: Thieme; 1976. So-called water immersion epilepsy; pp. 50–60. [Google Scholar]
- 17.Kurata S. Epilepsy precipitated by bathing: A follow up study. Brain Dev (Domestic ed) 1979;11:400–5. [Google Scholar]
- 18.Morimoto T, Hayakawa T, Sugie H, Awaya Y, Fukuyama Y. Epileptic seizures precipitated by constant light, movement in daily life, and hot water immersion. Epilepsia. 1985;26:237–42. doi: 10.1111/j.1528-1157.1985.tb05412.x. [DOI] [PubMed] [Google Scholar]
- 19.Gururaj G, Satishchandra P. Correlates of hot water epilepsy in rural south India: A descriptive study. Neuroepidemiology. 1992;11:173–9. doi: 10.1159/000110929. [DOI] [PubMed] [Google Scholar]
- 20.Mani KS, Rangan G, Srinivas HV, Kalyanasundaram S, Narendran S, Reddy AK. The Yelandur study: A community-based approach to epilepsy in rural South India - Epidemiological aspects. Seizure. 1998;7:281–8. doi: 10.1016/s1059-1311(98)80019-8. [DOI] [PubMed] [Google Scholar]
- 21.Engel J, Jr International League Against Epilepsy (ILAE) A proposed diagnostic scheme for people with epileptic seizures and with epilepsy: Report of the ILAE Task Force on Classification and Terminology. Epilepsia. 2001;42:796–803. doi: 10.1046/j.1528-1157.2001.10401.x. [DOI] [PubMed] [Google Scholar]
- 22.Meghana A, Sinha S, Sathyaprabha TN, Subbakrishna DK, Satishchandra P. Hot water epilepsy clinical profile and treatment - A prospective study. Epilepsy Res. 2012;102:160–6. doi: 10.1016/j.eplepsyres.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 23.Bebek N, Gürses C, Gokyigit A, Baykan B, Ozkara C, Dervent A, et al. Hot-water epilepsy: Clinical and electrophysiological findings based on 21 cases. Epilepsia. 2001;42:1130–4. doi: 10.1046/j.1528-1157.2001.31000.x. [DOI] [PubMed] [Google Scholar]
- 24.Roos RA, van Dijk JG. Reflex-epilepsy induced by immersion in hot water. Case report and review of the literature. Eur Neurol. 1988;28:6–10. doi: 10.1159/000116219. [DOI] [PubMed] [Google Scholar]
- 25.Shankar SK, Satishchandra P. Autopsy study of brains in hot water epilepsy. Neurology (India) 1994;42:56–7. [Google Scholar]
- 26.Klauenberg BJ, Sparber SB. A kindling-like effect induced by repeated exposure to heated water in rats. Epilepsia. 1984;25:292–301. doi: 10.1111/j.1528-1157.1984.tb04192.x. [DOI] [PubMed] [Google Scholar]
- 27.Satishchandra P, Ullal GR, Shankar SK. Experimental animal model for hot water epilepsy. Epilepsia. 1993;34(Suppl 2):101. [Google Scholar]
- 28.Ullal GR, Satishchandra P, Shankar SK. Hyperthermic seizures: An animal model for hot-water epilepsy. Seizure. 1996;5:221–8. doi: 10.1016/s1059-1311(96)80040-9. [DOI] [PubMed] [Google Scholar]
- 29.Ullal GR, Satishchandra P, Shankar SK. Seizure patterns, hippocampal and ictal temperature threshold with hyperthermic kindling in rats on hot-water stimulation. Epilepsia. 1995;36(Suppl 3):552. [Google Scholar]
- 30.Ullal GR, Satishchandra P, Shankar SK. Effect of antiepileptic drugs and calcium channel blocker on hyperthermic seizures in rats: Animal model for hot water epilepsy. Indian J Physiol Pharmacol. 1996;40:303–8. [PubMed] [Google Scholar]
- 31.Satishchandra P, Ullal GR, Shankar SK. Newer insight into the complexity of hot-water epilepsy. Epilepsia. 1995;36(Suppl 3):206–7. [Google Scholar]
- 32.Satishchandra P, Ullal GR, Shankar SK. Hot water epilepsy. In: Zifkin BG, Andermann F, Beaumanoir A, Rowan AJ, editors. Reflex Epilepsy and Reflex Seizures: Advances in Neurology. Philadelphia: Lippincott-Raven; 1998. pp. 283–94. [Google Scholar]
- 33.Meghana A, Sathyaprabha TN, Sinha S, Satishchandra P. Cardiac autonomic dysfunction in drug naïve hot water epilepsy. Seizure. 2012;21:706–10. doi: 10.1016/j.seizure.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 34.Meghana A. PhD Thesis Submitted. NIMHANS. 2013. Characterization of Autonomic Functions in Reflex and Non-Reflex Epilepsy. [Google Scholar]
- 35.Satishchandra P, Kallur KG, Jayakumar PN. Inter-ictal and ictal 99 m TC ECD SPECT scan in hot-water epilepsy. Epilepsia. 2001;42(Suppl):S1–158. [Google Scholar]
- 36.Patel M. DM (neurology) PhD Dissertation. NIMHANS. 2012. Phenotypic, MR imaging, ictal and inter-ictal SPECT and video-EEG observations in patients with Reflex epilepsy. [Google Scholar]
- 37.Satishchandra P, Ullal GR, Sinha A, Shankar SK. Pathophysiology and genetics of hot-water epilepsy. In: Bercovic S, Genton P, Marescaux C, Picard F, editors. Genetics of Focal Epilepsies: Clinical Aspects and Molecular Biology. London: John Libbey & Company Ltd.; 1999. pp. 169–76. [Google Scholar]
- 38.Sinha A, Ullal GR, Shankar SK, Satishchandra P, et al. Genetics of hot-water epilepsy: A preliminary analysis. Curr Sci. 1999;77:1407–10. [Google Scholar]
- 39.Ratnapriya R, Satishchandra P, Kumar SD, Gadre G, Reddy R, Anand A. A locus for autosomal dominant reflex epilepsy precipitated by hot water maps at chromosome 10q21.3-q22.3. Hum Genet. 2009;125:541–9. doi: 10.1007/s00439-009-0648-3. [DOI] [PubMed] [Google Scholar]
- 40.Ratnapriya R, Satishchandra P, Dilip S, Gadre G, Anand A. Familial autosomal dominant reflex epilepsy triggered by hot water maps to 4q24-q28. Hum Genet. 2009;126:677–83. doi: 10.1007/s00439-009-0718-6. [DOI] [PubMed] [Google Scholar]
- 41.Audenaert D, Van Broeckhoven C, De Jonghe P. Genes and loci involved in febrile seizures and related epilepsy syndromes. Hum Mutat. 2006;27:391–401. doi: 10.1002/humu.20279. [DOI] [PubMed] [Google Scholar]
- 42.Jackson FR, Wilson SD, Strichartz GR, Hall LM. Two types of mutants affecting voltage-sensitive sodium channels in Drosophila melanogaster. Nature. 1984;308:189–91. doi: 10.1038/308189a0. [DOI] [PubMed] [Google Scholar]
- 43.Wang P, Saraswati S, Guan Z, Watkins CJ, Wurtman RJ, Littleton JT. A Drosophila temperature-sensitive seizure mutant in phosphoglycerate kinase disrupts ATP generation and alters synaptic function. J Neurosci. 2004;24:4518–29. doi: 10.1523/JNEUROSCI.0542-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]


