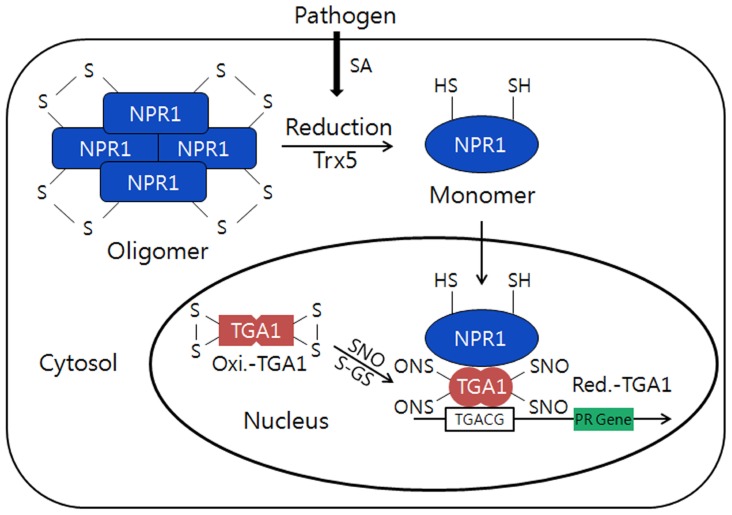
FIGURE 3.
Conformational switching from the oligomers to a monomer of NPR1 by redox changes in plants. Under normal conditions, NPR1forms an inactive oligomer structure and is oxidized by intermolecular disulfide bonds in the cytosol. However, during pathogen attack, the NPR1 changes its structure from inactive to active form of monomer through the reduction of the intermolecular bridges by h5-type thioredoxin (AtTrx-h5). Then, NPR1 will be translocated to the nucleus and interacts withTGA1transcription factor that can induce PR gene expression. TGA1 in normal condition is inactive and oxidized form with intramolecular disulfide bonds in the nucleus. However, in stress conditionTGA1 will be reduced and interact with NPR1 and can enhance its DNA binding activity through S-nitrosylation (SNO) and S-glutathionylation (S-GS) by GSNO and glutathione (GSH/GSSG). This model is modified from the reference of Moore et al. (2011).