Sir
Transbronchial needle aspiration (TBNA) has been shown to be an effective and safe technique in the diagnosis of intrathoracic lymphadenopathy1,2,3. However, the steep learning curve, faulty techniques, unfounded concerns regarding the safety of the procedure has discouraged pulmonologists to perform this as a routine procedure4. A survey of pulmonary specialists in North America revealed that only 11.8 per cent of the responding pulmonary specialists routinely used TBNA while 49.4 per cent rarely used the procedure5. A subsequent study again demonstrated the infrequent use of TBNA with 54 per cent performing TBNA in the preceding 12 months in less than 10 instances and 18 per cent never utilizing TBNA in the prior year6. In a study from the United Kingdom, which reviewed the responses of 328 consultants in respiratory medicine, found that TBNA was being utilized by only 27 per cent of practitioners in the preceding 12 months due to its poor diagnostic yield7. The scenario has however, changed in the last decade. In a recent article with our experience of 473 TBNA procedures, we showed that the yield of TBNA improved with experience8. TBNA has a variable sensitivity ranging from 20-89 per cent depending on the aetiology, size and location of the lymph node, and the operator's experience4,9,10,11,12,13. Despite five years of performing TBNA, the sensitivity of conventional TBNA without an on-site cytopathologist was only 40 per cent in our study. Hence, there is a need for additional modality to improve the yield of TBNA. Endobronchial ultrasound (EBUS) can improve the diagnostic yield of TBNA by enabling visualization of the lymph nodes beyond the tracheal or bronchial wall, and with the advent of the convex probe it allows real time sampling of the mediastinal and hilar lymph nodes14. Since the first description of EBUS-TBNA in 200315, there is no report from India describing experience with this procedure. In this study, we report our initial experience with EBUS-TBNA in patients presenting with mediastinal lymphadenopathy of unknown aetiology in a tertiary care hospital in north India.
A prospective review of all consecutive EBUS-TBNA procedures performed at the department of Pulmonary Medicine, Postgraduate Institute of Medical Education & Research, Chandigarh, over three months period (July 15 to October 15, 2011) was done. The study was approved by the institutional Ethics Committee and an informed consent was obtained from all patients. All patients undergoing diagnostic bronchoscopy for intrathoracic lymphadenopathy identified on computed tomography (CT) scan of the chest, irrespective of the aetiology, were offered the option of EBUS-TBNA. Patients not willing for EBUS-TBNA due to the cost associated with the dedicated EBUS-TBNA needle underwent conventional TBNA. A detailed history, physical examination, radiological findings and provisional clinical diagnosis were recorded. Lymph nodes were considered enlarged and amenable for TBNA when the short axis nodal diameter on CT chest was >1 cm, and were classified using the Association for the Study of Lung Cancer (IASLC) classification16. All patients received nebulized lignocaine (4% solution) immediately before the procedure, coupled with injection of 0.6 mg atropine and 25 mg promethazine intramuscularly. Intravenous midazolam was used as the agent for sedation and pentazocine for analgesia, and the total dose required was noted in each patient. Topical 10 per cent lignocaine spray was applied in the oropharynx augmented with ‘spray-as-you-go’ 2 per cent lignocaine over the vocal cords and the airways. Bronchoscopy was done on an outpatient basis using the EBUS scope (BF-UC 180F; Olympus Medical Systems, Singapore) with a compatible endoscopic ultrasound unit (EU-ME1; Olympus Medical Systems, Singapore). The procedure was done in the supine position through the oral route. The dimension of the lymph nodes were documented by measuring the short axis diameter of the nodes on EBUS once the best view of the node was identified. All TBNA specimens were obtained using a dedicated, disposable, 21-gauge, Vizishot needle (NA-201SX-4021 Olympus Medical Systems, Singapore) using the jabbing method under real-time ultrasound control. The same needle was used for all stations as the aim of EBUS-TBNA in this study was only diagnostic. We used 21G instead of 22G due to proposed better preservation of histological structure of the samples with the larger needle17. Continuous suction was applied with a dedicated 20 ml syringe while the catheter was moved back and forth for up to a maximum of 20 times. A maximum of three aspirates were obtained from each location; only two aspirates were obtained if a core biopsy specimen was identified18. In case of multiple lymph node enlargements, the most enlarged lymph nodes were accessed. On-site cytological assessment for adequacy of the sample was not available. Secretions and cough during the procedure was rated by the operator using a visual analog scale (VAS) from 0-100 millimeters. Bronchoalveolar lavage (BAL), transbronchial biopsy (TBB) and endobronchial biopsy (EBB) were also done as clinically indicated using the conventional flexible bronchoscope. An experienced cytopathologist examined all the smears for adequacy of samples and definite diagnosis. Adequate lymph node samples were defined by preponderance of benign lymphocytes, and represented a successful procedure. Malignancy was diagnosed based on the representative samples containing malignant cells. In the presence of compatible clinicoradiological features, compact epithelioid cell granuloma without necrosis and negative acid-fast bacilli (AFB) smears was considered diagnostic of sarcoidosis. Smears were considered diagnostic of tuberculosis (TB) in the presence of extensive necrotizing granulomas and/or demonstration of AFB. In patients with suspected TB, the aspirates were also sent for mycobacterial cultures by the mycobacterial growth indicator tube technique. All patients with negative EBUS are given an option to undergo CT-guided fine needle aspiration cytology, and were followed up for six months to determine the actual diagnosis, however, no patient underwent mediastinoscopy.
A total of 483 bronchoscopies were done during the study period, and 46 patients (9.5%) were considered eligible for TBNA. Of these, 39 patients (8.1%) underwent EBUS-TBNA while the remaining underwent conventional TBNA. There were 23 men and 16 women with a median age of 43 yr. The most common clinical diagnosis was sarcoidosis followed by tuberculosis and lung cancer (Table I). A total of 71 nodes were sampled in 39 patients. The median number of lymph node groups enlarged on CT was two, and a median of two lymph node sites were accessed in every patient (Table II). Adequate lymph node sampling (i.e. presence of lymphocytes) was obtained in 37 patients while a diagnostic sample was obtained in 27(69.2%) patients (Table II). The diagnostic yield increased to 73 per cent (27 of 37) after excluding cases without adequate lymph node sampling. Of the 21 patients suspected to have sarcoidosis, three were diagnosed as tuberculosis based on EBUS. Of the 18 patients, in two patients with suspected sarcoidosis EBUS-TBNA was representative but no granulomas could be demonstrated in EBUS, TBB and EBB. Both the patients were followed up without treatment for 6 months. There was no enlargement of lymph nodes and no evidence of sarcoid activity at any other site, and thus were deemed to have idiopathic mediastinal adenopathy. Thus, EBUS cytology was able to establish diagnosis of sarcoidosis in 12 of 16 patients. TBB and EBB enabled diagnosis in four additional patients where EBUS was non-contributory. EBUS was able to establish a diagnosis of TB in nine of 14 patients, of whom two were positive for AFB and four were culture positive on EBUS aspirate. Of the remaining five patients, two were diagnosed on CT guided FNAC while the lymph nodes of three patients resolved after an empiric trial of anti-tuberculosis therapy. The sensitivity of EBUS-TBNA was 85.7 per cent in patients with a clinical diagnosis of lung cancer (Table III). One patient negative on EBUS was diagnosed to have squamous cell carcinoma on CT-guided FNAC. Most patients tolerated the procedure with median (IQR) VAS for secretions and cough being 19 and 24 mm, respectively. The median dose of midazolam was 8 mg per procedure, and the median duration of procedure was 40 min (Table II). Minor complications were encountered in four patients (10.3%) which included post procedure chills in two patients, prolonged sedation in one and minor bleed with hypoxemia requiring high flow oxygen supplementation through Venturi mask in one patient. However, none required admission for the complications.
Table I.
Baseline characteristics of 39 patients with mediastinal lymphadenopathy who underwent real-time EBUS-TBNA
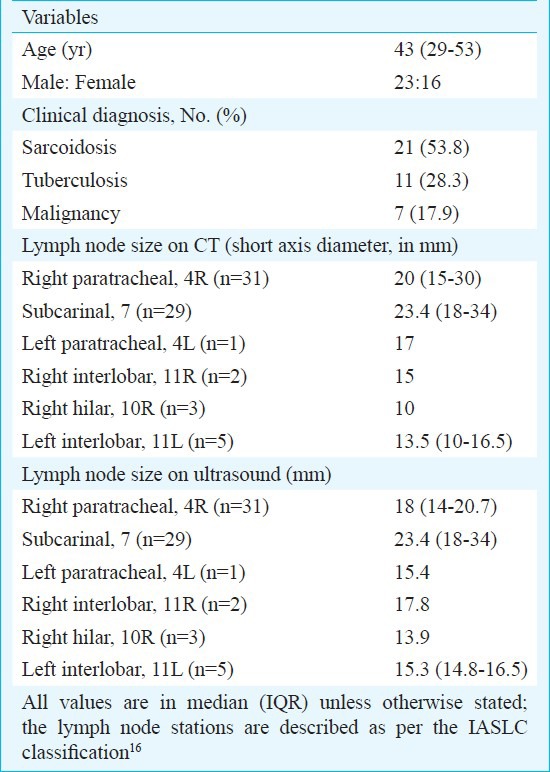
Table II.
Procedural details of patients undergoing EBUS-TBNA
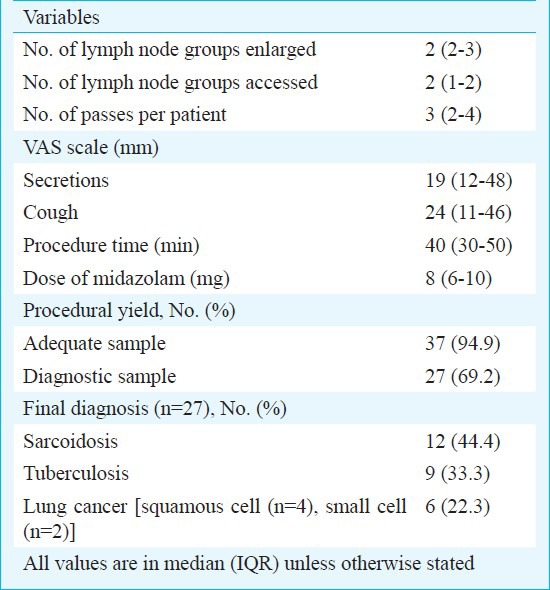
Table III.
Sensitivity and specificity of EBUS-TBNA in mediastinal lymphadenopathy
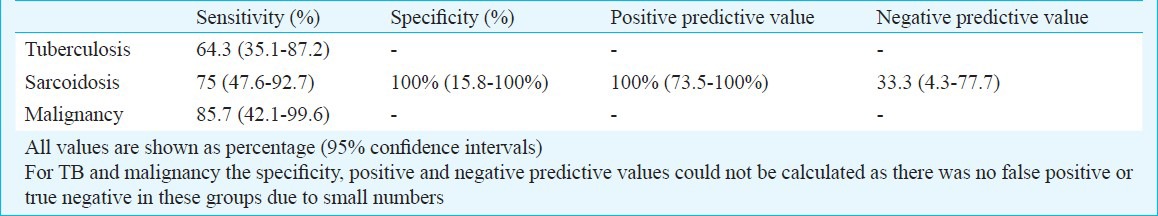
Diagnosis of mediastinal lymphadenopathy remains a challenge for respiratory physicians. We were able to achieve representative samples in 94.9 per cent of patients, and confirmation of diagnosis was established in 69.2 per cent. Other studies have reported higher diagnostic yield, ranging from 93.5-100 per cent19,20,21,22,23. These studies have evaluated suspected mediastinal metastasis of bronchogenic carcinoma or diagnosis of suspected malignancy. In our study, the diagnostic yield of EBUS in patients with clinical suspicion of malignancy was 85.7 per cent and was in agreement with a recent systematic review evaluating the role of EBUS in patients with suspected malignancy24. Our results were comparable to studies evaluating learning curve and early experience in centers starting an EBUS-TBNA programme evaluating enlarged lymph nodes irrespective of aetiology, where the diagnostic yield has ranged from 63-67 per cent25,26,27. However, the target should be a sensitivity of 88-90 per cent for malignancy28. The sensitivity of EBUS in sarcoidosis was 75 per cent and was better than the yield of conventional TBNA (38.1%) reported from our center8. The sensitivity of EBUS-TBNA in sarcoidosis has ranged from 50-97 per cent27,29,30,31,32, and the lower diagnostic yield in our study is likely due to the small sample size, less number of passes per node and the learning curve associated with the procedure. In patients with suspected tuberculosis, the sensitivity of EBUS was 64.3 per cent even after smear and culture. Additionally, EBUS diagnosed TB in three patients who were initially thought to be sarcoidosis on clinical grounds. The yield of EBUS-TBNA in TB has been reported to be 94 per cent in a recent study33. The lower yield apart from the learning curve and non-availability of rapid on-site cytological evaluation, is possibly due to the fact that several of our patients had been started on anti tuberculous therapy on the basis of clinical suspicion. Another reason could be lack of performance of systematic examination of nodes from proximal to distal stations regardless of nodal size on CT. As the aim of EBUS-TBNA in this study was only diagnostic, the largest lymph nodes identified on CT were sampled. The patients were followed only for 6 months after a negative EBUS-TBNA leading to a risk of missing other pathologies which appeared after 6 months. Also not being able to validate negative EBUS-TBNA with mediastinoscopy (which was not available on- site) was another limitation.
In conclusion, EBUS-TBNA may be a promising tool for interventional pulmonologist enabling minimally invasive access to mediastinal nodes under real-time guidance. Our preliminary experience suggested that the sensitivity of EBUS-TBNA for diagnosing of intrathoracic lymphadenopathy was better than conventional TBNA.
Acknowledgment
This work was supported by a FIST grant from the Department of Science & Technology, New Delhi, India.
References
- 1.Holty JE, Kuschner WG, Gould MK. Accuracy of transbronchial needle aspiration for mediastinal staging of non-small cell lung cancer: a meta-analysis. Thorax. 2005;60:949–55. doi: 10.1136/thx.2005.041525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Detterbeck FC, Jantz MA, Wallace M, Vansteenkiste J, Silvestri GA. Invasive mediastinal staging of lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition) Chest. 2007;132(3 Suppl):202S–20S. doi: 10.1378/chest.07-1362. [DOI] [PubMed] [Google Scholar]
- 3.Medford AR, Agrawal S, Free CM, Bennett JA. A prospective study of conventional transbronchial needle aspiration: performance and cost utility. Respiration. 2010;79:482–9. doi: 10.1159/000277931. [DOI] [PubMed] [Google Scholar]
- 4.Dasgupta A, Mehta AC. Transbronchial needle aspiration. An underused diagnostic technique. Clin Chest Med. 1999;20:39–51. doi: 10.1016/s0272-5231(05)70125-8. [DOI] [PubMed] [Google Scholar]
- 5.Prakash UB, Offord KP, Stubbs SE. Bronchoscopy in North America: the ACCP survey. Chest. 1991;100:1668–75. doi: 10.1378/chest.100.6.1668. [DOI] [PubMed] [Google Scholar]
- 6.Colt HG, Prakash UBS, Offord KP. Bronchoscopy in North America: Survey by the American Association for Bronchology, 1999. J Bronchology Interv Pulmonol. 2000;7:8–25. [Google Scholar]
- 7.Smyth CM, Stead RJ. Survey of flexible fibreoptic bronchoscopy in the United Kingdom. Eur Respir J. 2002;19:458–63. doi: 10.1183/09031936.02.00103702. [DOI] [PubMed] [Google Scholar]
- 8.Khan A, Agarwal R, Aggarwal AN, Gupta N, Bal A, Singh N, et al. Blind transbronchial needle aspiration without an on-site cytopathologist: experience of 473 procedures. Natl Med J India. 2011;24:136–9. [PubMed] [Google Scholar]
- 9.Harrow EM, Oldenburg FA, Smith AM. Transbronchial needle aspiration in clinical practice. Thorax. 1985;40:756–9. doi: 10.1136/thx.40.10.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schenk DA, Bower JH, Bryan CL, Currie RB, Spence TH, Duncan CA, et al. Transbronchial needle aspiration staging of bronchogenic carcinoma. Am Rev Respir Dis. 1986;134:146–8. doi: 10.1164/arrd.1986.134.1.146. [DOI] [PubMed] [Google Scholar]
- 11.Harrow EM, Oldenburg FA, Jr, Lingenfelter MS, Smith AM., Jr Transbronchial needle aspiration in clinical practice A five-year experience. Chest. 1989;96:1268–72. doi: 10.1378/chest.96.6.1268. [DOI] [PubMed] [Google Scholar]
- 12.Gay PC, Brutinel WM. Transbronchial needle aspiration in the practice of bronchoscopy. Mayo Clin Proc. 1989;64:158–62. doi: 10.1016/s0025-6196(12)65669-9. [DOI] [PubMed] [Google Scholar]
- 13.Haponik EF, Shure D. Underutilization of transbronchial needle aspiration: experiences of current pulmonary fellows. Chest. 1997;112:251–3. doi: 10.1378/chest.112.1.251. [DOI] [PubMed] [Google Scholar]
- 14.Burgers JA, Herth F, Becker HD. Endobronchial ultrasound. Lung Cancer. 2001;34(Suppl 2):S109–13. doi: 10.1016/s0169-5002(01)00349-x. [DOI] [PubMed] [Google Scholar]
- 15.Krasnik M, Vilmann P, Larsen SS, Jacobsen GK. Preliminary experience with a new method of endoscopic transbronchial real time ultrasound guided biopsy for diagnosis of mediastinal and hilar lesions. Thorax. 2003;58:1083–6. doi: 10.1136/thorax.58.12.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rusch VW, Asamura H, Watanabe H, Giroux DJ, Rami-Porta R, Goldstraw P. The IASLC lung cancer staging project: a proposal for a new international lymph node map in the forthcoming seventh edition of the TNM classification for lung cancer. J Thorac Oncol. 2009;4:568–77. doi: 10.1097/JTO.0b013e3181a0d82e. [DOI] [PubMed] [Google Scholar]
- 17.Nakajima T, Yasufuku K, Takahashi R, Shingyoji M, Hirata T, Itami M, et al. Comparison of 21-gauge and 22-gauge aspiration needle during endobronchial ultrasound-guided transbronchial needle aspiration. Respirology. 2011;16:90–4. doi: 10.1111/j.1440-1843.2010.01871.x. [DOI] [PubMed] [Google Scholar]
- 18.Lee HS, Lee GK, Kim MS, Lee JM, Kim HY, Nam BH, et al. Real-time endobronchial ultrasound-guided transbronchial needle aspiration in mediastinal staging of non-small cell lung cancer: how many aspirations per target lymph node station? Chest. 2008;134:368–74. doi: 10.1378/chest.07-2105. [DOI] [PubMed] [Google Scholar]
- 19.Herth FJ, Eberhardt R, Vilmann P, Krasnik M, Ernst A. Real-time endobronchial ultrasound guided transbronchial needle aspiration for sampling mediastinal lymph nodes. Thorax. 2006;61:795–8. doi: 10.1136/thx.2005.047829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bauwens O, Dusart M, Pierard P, Faber J, Prigogine T, Duysinx B, et al. Endobronchial ultrasound and value of PET for prediction of pathological results of mediastinal hot spots in lung cancer patients. Lung Cancer. 2008;61:356–61. doi: 10.1016/j.lungcan.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 21.Yasufuku K, Chiyo M, Koh E, Moriya Y, Iyoda A, Sekine Y, et al. Endobronchial ultrasound guided transbronchial needle aspiration for staging of lung cancer. Lung Cancer. 2005;50:347–54. doi: 10.1016/j.lungcan.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 22.Yasufuku K, Nakajima T, Motoori K, Sekine Y, Shibuya K, Hiroshima K, et al. Comparison of endobronchial ultrasound, positron emission tomography, and CT for lymph node staging of lung cancer. Chest. 2006;130:710–8. doi: 10.1378/chest.130.3.710. [DOI] [PubMed] [Google Scholar]
- 23.Vincent BD, El-Bayoumi E, Hoffman B, Doelken P, DeRosimo J, Reed C, et al. Real-time endobronchial ultrasound-guided transbronchial lymph node aspiration. Ann Thorac Surg. 2008;85:224–30. doi: 10.1016/j.athoracsur.2007.07.023. [DOI] [PubMed] [Google Scholar]
- 24.Varela-Lema L, Fernandez-Villar A, Ruano-Ravina A. Effectiveness and safety of endobronchial ultrasound-transbronchial needle aspiration: a systematic review. Eur Respir J. 2009;33:1156–64. doi: 10.1183/09031936.00097908. [DOI] [PubMed] [Google Scholar]
- 25.Tian Q, Chen LA, Wang HS, Zhu BH, Tian L, Yang Z, et al. Endobronchial ultrasound-guided transbronchial needle aspiration of undiagnosed mediastinal lymphadenopathy. Chin Med J (Engl) 2010;123:2211–4. [PubMed] [Google Scholar]
- 26.Arslan Z, Ilgazli A, Bakir M, Yildiz K, Topcu S. Conventional vs. endobronchial ultrasound-guided transbronchial needle aspiration in the diagnosis of mediastinal lymphadenopathies. Tuberk Toraks. 2011;59:153–7. doi: 10.5578/tt.2403. [DOI] [PubMed] [Google Scholar]
- 27.Jernlas B, Nyberger H, Ek L, Ohman R, Jonsson P, Nozohoor S. Diagnostic yield and efficacy of endobronchial ultrasound-guided transbronchial needle aspiration in mediastinal lymphadenopathy. Clin Respir J. 2012;6:88–95. doi: 10.1111/j.1752-699X.2011.00251.x. [DOI] [PubMed] [Google Scholar]
- 28.Medford AR, Agrawal S, Free CM, Bennett JA. A performance and theoretical cost analysis of endobronchial ultrasound-guided transbronchial needle aspiration in a UK tertiary respiratory centre. QJM. 2009;102:859–64. doi: 10.1093/qjmed/hcp136. [DOI] [PubMed] [Google Scholar]
- 29.Wong M, Yasufuku K, Nakajima T, Herth FJ, Sekine Y, Shibuya K, et al. Endobronchial ultrasound: new insight for the diagnosis of sarcoidosis. Eur Respir J. 2007;29:1182–6. doi: 10.1183/09031936.00028706. [DOI] [PubMed] [Google Scholar]
- 30.Oki M, Saka H, Kitagawa C, Tanaka S, Shimokata T, Kawata Y, et al. Real-time endobronchial ultrasound-guided transbronchial needle aspiration is useful for diagnosing sarcoidosis. Respirology. 2007;12:863–8. doi: 10.1111/j.1440-1843.2007.01145.x. [DOI] [PubMed] [Google Scholar]
- 31.Garwood S, Judson MA, Silvestri G, Hoda R, Fraig M, Doelken P. Endobronchial ultrasound for the diagnosis of pulmonary sarcoidosis. Chest. 2007;132:1298–304. doi: 10.1378/chest.07-0998. [DOI] [PubMed] [Google Scholar]
- 32.Cetinkaya E, Gunluoglu G, Ozgul A, Gunluoglu MZ, Ozgul G, Seyhan EC, et al. Value of real-time endobronchial ultrasound-guided transbronchial needle aspiration. Ann Thorac Med. 2011;6:77–81. doi: 10.4103/1817-1737.78422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Navani N, Molyneaux PL, Breen RA, Connell DW, Jepson A, Nankivell M, et al. Utility of endobronchial ultrasound-guided transbronchial needle aspiration in patients with tuberculous intrathoracic lymphadenopathy: a multicentre study. Thorax. 2011;66:889–93. doi: 10.1136/thoraxjnl-2011-200063. [DOI] [PMC free article] [PubMed] [Google Scholar]


