Fig. 6.
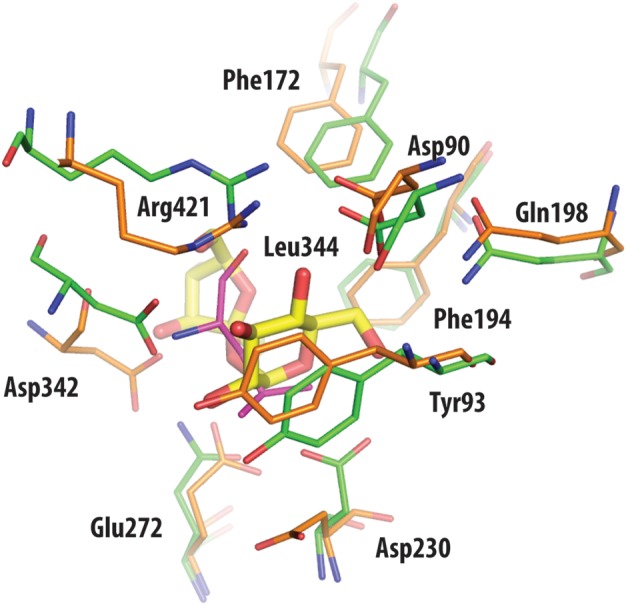
Superposition of active site residues of TreS (orange) and trehalulose synthase MutB (green), with residue numbering according to the TreS structure. With the exception of the side chain of the nucleophilic Asp230 in TreS, other catalytic residues are in comparable positions between the superimposed structures. Also drawn in yellow is the disaccharide substrate sucrose of MutB, as found bound in the active site of this enzyme. Leu344 of TreS (shown in magenta) overlaps with the sucrose molecule of MutB, hence prohibiting ligand access to the catalytic Asp230.
