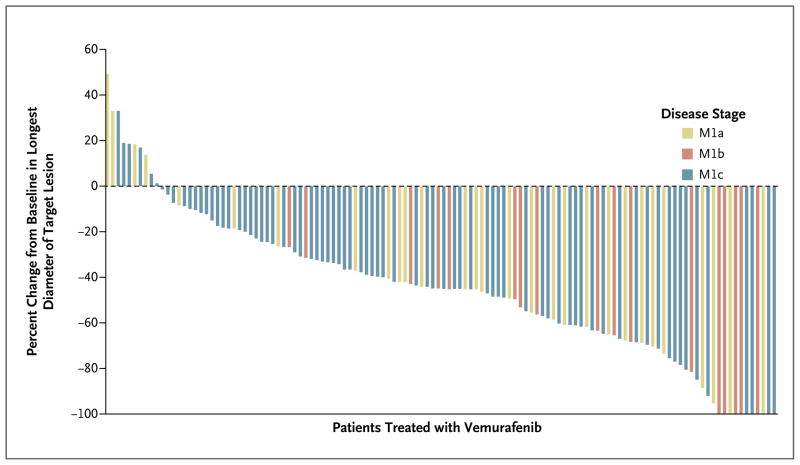
Figure 1. Objective Tumor Responses with Vemurafenib, According to Metastatic Stage.
Ten patients had 100% reduction in target lesions; two of these had nontarget lesions and were therefore considered to have a partial response, for a total of eight complete responses as defined on the basis of the Response Evaluation Criteria in Solid Tumors (version 1.1). Up to five measurable target lesions (no more than two per organ) were selected to assess response. A complete response was defined as the disappearance of all target lesions and nontarget lesions. A partial response was defined as a decrease of at least 30% in the sum of the diameters of target lesions, as compared with the baseline sum of the diameters.