Scheme 1.
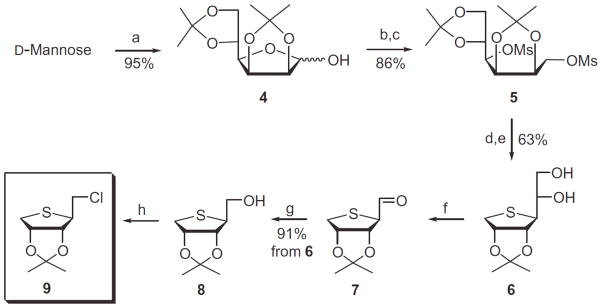
Reagents and conditions: (a) 2,2-dimethoxypropane, camphosulfonic acid, acetone, rt; (b) NaBH4, EtOH, rt; (c) MsCl, Et3N, CH2C12, rt; (d) Na2S, DMF, 80 °C; (e) 60% AcOH, rt; (f) Pb(OAc)4, EtOAc, 0 °C; (g) NaBH4, EtOH, 0 °C; (h) POCl3, CH3CN, rt.
