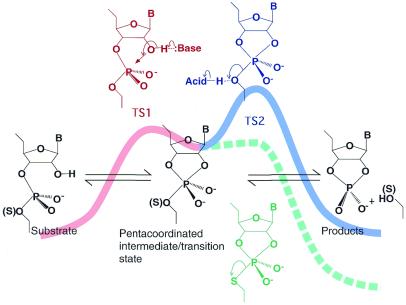
Figure 2.
The two-step reaction scheme for the hydrolysis of a phosphodiester bond in RNA. First, the 2′-oxygen attacks the phosphorus atom, acting as an internal nucleophile, to generate the pentacoordinated intermediate or transtion state TS1. The 5′-oxygen then departs from the intermediate to complete cleavage at TS2. TS1 can be stabilized by a general base catalyst and TS2 can be stabilized by a general acid catalyst, as illustrated at the summits of the energy diagram. These transition states can also be stabilized by the direct binding of Lewis acids to the 2′-attacking oxygen and the 5′-leaving oxygen.