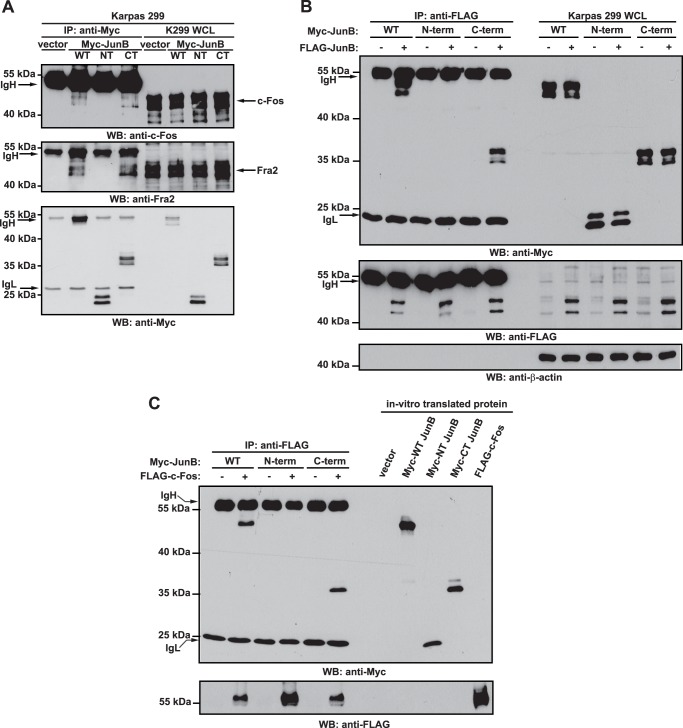
FIGURE 6.
The C-terminal JunB cleavage fragment can co-precipitate with c-Fos, Fra2, and full-length JunB. A, Karpas 299 cells were transfected with vector alone or cDNAs encoding for Myc-tagged JunB (WT), Myc-tagged N-terminal JunB cleavage fragment (NT), or Myc-tagged C-terminal JunB cleavage fragment (CT). Twenty-four h after transfection, cells were lysed, and lysates were immunoprecipitated (IP) with an anti-Myc antibody. Western blotting (WB) was then performed on immunoprecipitates with anti-c-Fos, anti-Fra2, or anti-Myc antibodies. B, Karpas 299 cells were transfected with the cDNAs encoding for the indicated proteins. Cells were lysed 24 h after transfection, and anti-FLAG immunoprecipitation was performed on lysates. Western blotting was then performed on the immunoprecipitates. C, the indicated in vitro transcribed/translated Myc-tagged JunB proteins were incubated with in vitro transcribed/translated FLAG-c-Fos, and anti-FLAG immunoprecipitations (IP) were performed. Western blotting (WB) was then performed on the immunoprecipitates. Cell lysates (A and B) or TNT extracts (C) were run to show the electrophoretic mobility and the presence of the respective proteins. The immunoglubulin heavy (IgH) and light chains (IgL) of the immunoprecipitating antibody are indicated. The electrophoretic mobility of molecular mass standards is indicated to the left of blots.