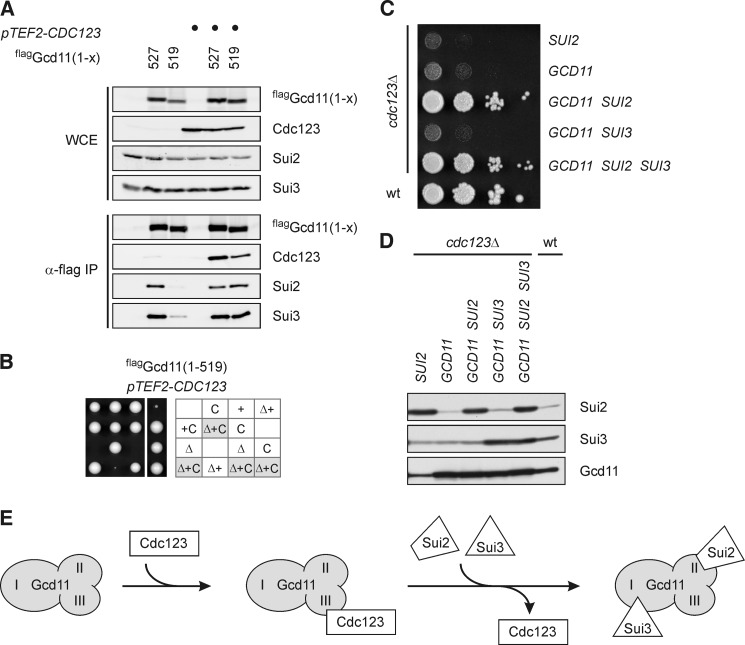
FIGURE 5.
eIF2 complex formation is the essential function of Cdc123. A and B, rescue of Gcd11-(1–519) by overexpression of CDC123. Full-length flag-Gcd11-(1–527) or the truncated version flag-Gcd11(1–519) in the absence or presence of overexpressed Cdc123 (pTEF2-CDC123) were immunoprecipitated and analyzed for interaction of Cdc123, Sui2, and Sui3. Protein levels in WCE and anti-flag immunoprecipitates (α-flag IP) were determined by Western analysis (A). A gcd11Δ/GCD11 heterozygous diploid strain carrying the flag-GCD11-(1–519) and pTEF-CDC123 constructs was sporulated and meiotic progeny was analyzed by tetrad dissection. Δ, gcd11Δ; +, flag-GCD11(1–519); C, pTEF-CDC123 (B). C and D, rescue of a cdc123 deletion mutant by overexpression of eIF2 subunits. Cells were spotted in serial dilutions on an agar plate, and growth was monitored at 30 °C (C). Overexpression of eIF2 subunits was confirmed by Western blotting (D). E, model of Cdc123 function. Through binding to domain III of Gcd11, Cdc123 promotes association of Gcd11 with Sui2 and Sui3, i.e. formation of the heterotrimeric eIF2 protein complex.