FIGURE 1.
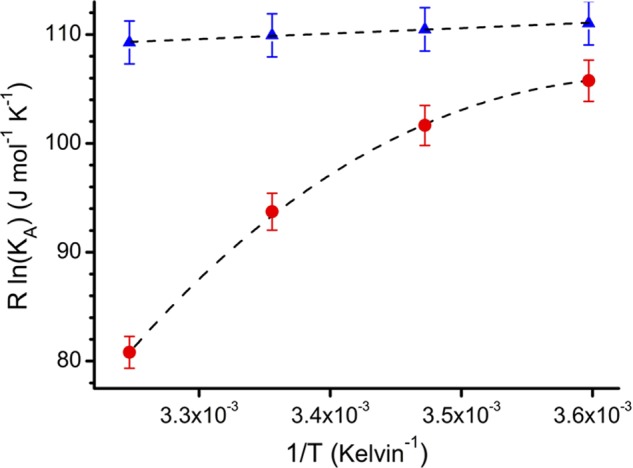
Temperature dependence of the derCD23/IgE-Fc interaction in the presence and absence of Ca2+. For Ca2+-bound and Ca2+-free states, binding affinities were measured by surface plasmon resonance at 5, 15, 25, and 35 °C; affinities at these temperatures for the Ca2+-bound states were 1.6, 1.7, 1.8, and 2.0 μm, respectively, and for the Ca2+-free state 3.0, 4.4, 10.2, and 58.0 μm, respectively. Data are shown as a van't Hoff plot (47) and were fitted to linear or nonlinear integrated forms of the van't Hoff equation (48). Ca2+-bound derCD23 (blue triangles) binds with a higher affinity than Ca2+-free derCD23 (red circles) to IgE-Fc. Ca2+-bound derCD23 shows small decreases in affinities at increasing temperatures, indicating a small favorable enthalpic contribution to binding energy (ΔH = −5.1 kJ mol−1). Ca2+-free derCD23 shows a larger temperature dependence, an indication of a larger contribution from enthalpy to the binding event (ΔH = −33.2 kJ mol−1); this interaction also shows a nonlinear temperature dependence, characteristic of an associated heat capacity change (ΔCp).
