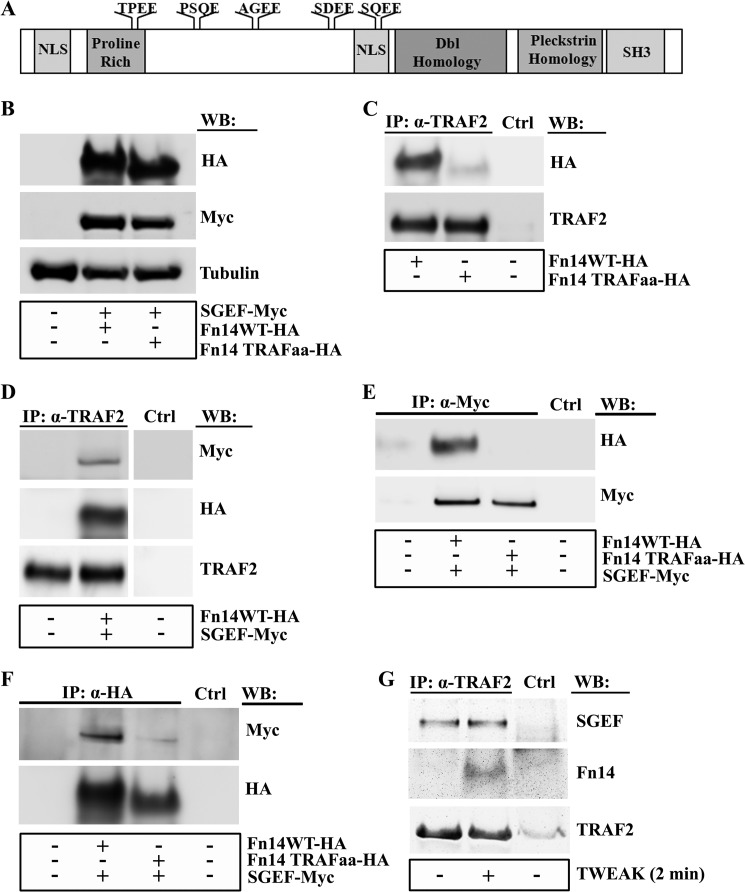
FIGURE 6.
SGEF and Fn14 interaction is dependent upon a functional TRAF domain. A, the SGEF protein contains five TRAF2 binding consensus motifs of the pattern (P/S/A/T/)X(Q/E)E, including TPEE (aa 157–160), PSQE (aa 221–224), AGEE (aa 292–295), SDEE (aa 392–395), and SQEE (aa 434–437). NLS, nuclear localization sequence. B, whole cell lysates of HEK293 cells transiently transfected with plasmids encoding Fn14wt-HA, Fn14TRAFaa-HA, or SGEF-myc were immunoblotted (WB) as indicated. C, whole cell lysates of HEK293 cells transiently transfected with plasmids encoding Fn14wt-HA or Fn14TRAFaa were collected and precleared, followed by immunoprecipitation (IP) using antibodies as indicated for anti-TRAF2 or control immunoprecipitation (Ctrl) and resolution via SDS-PAGE analysis using an anti-HA antibody. D, whole cell lysates of HEK293 cells transiently transfected with plasmids encoding Fn14wt-HA or SGEF-myc were collected and precleared, followed by immunoprecipitation using antibodies as indicated for anti-TRAF2 or control immunoprecipitation (Ctrl) and resolution via SDS-PAGE analysis using anti-myc and anti-HA antibodies. E and F, HEK293 cells transiently transfected with plasmids encoding Fn14wt-HA, Fn14TRAFaa, or SGEF-myc were collected and precleared, followed by immunoprecipitation using antibodies as indicated for anti-myc (E), anti-HA (F), or control immunoprecipitation (Ctrl) and resolution via SDS-PAGE analysis using an anti-HA (E) or anti-myc (F) antibody. G, U87 glioma cells were cultured for 16 h in reduced-serum (0.5% FBS) medium. Cells were either left untreated or treated with TWEAK for 2 min, after which whole cell lysates were precleared, followed by immunoprecipitation using antibodies indicated for anti-TRAF2 or control immunoprecipitation (Ctrl) and resolution via SDS-PAGE analysis using anti-SGEF and anti-Fn14 antibodies.