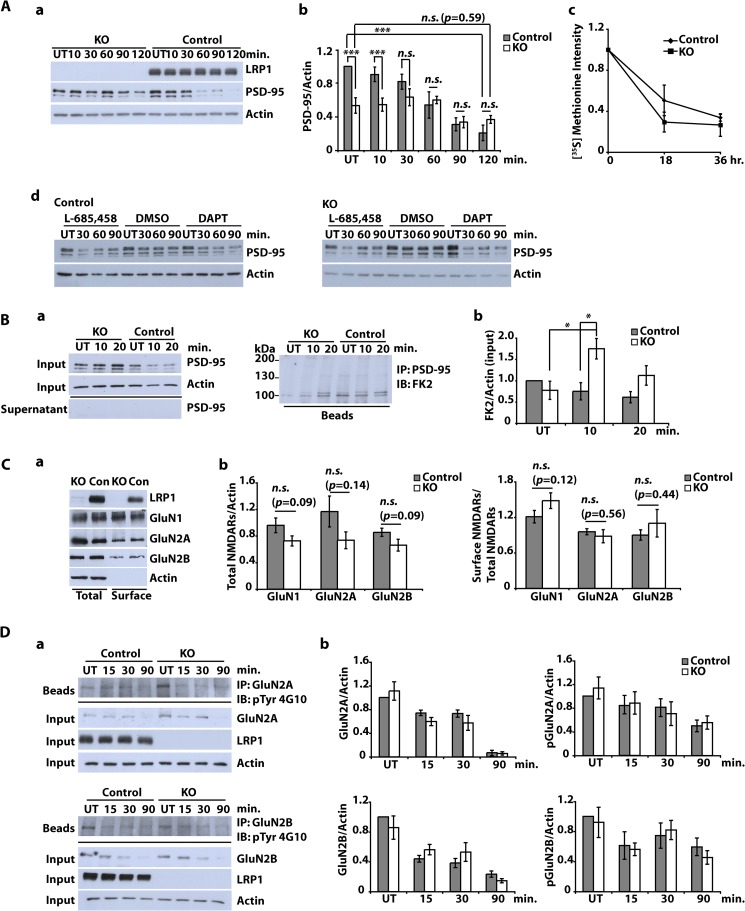
FIGURE 5.
Subcellular localization of NMDA receptor subunits and NMDA-dependent PSD-95 degradation in LRP1-deficient neurons. A (a and b), primary cultured cortical neurons were prepared from E15–E16 NesCreLRP1lox/lox (KO) and E15 LRP1wt/lox (Control) mouse embryos. On DIV 10–12, neurons were treated with NMDA (20 μm, 3 min), and whole cell lysates were prepared after the time indicated. Immunoblot analysis was performed with α-LRP1 and α-PSD-95 antibodies. Actin served as a loading control. Representative blot (a) and quantitative analysis (b) of six independent experiments are shown. Error bars, S.E. Statistical analysis was done by Student's t test (***, p < 0.001). c, cortical neurons were cultured from NesCreLRP1lox/lox (KO) and LRP1wt/lox (Control) mouse embryos and pulse-labeled with [35S]methionine on DIV 14–16. The cultures were chased with unlabeled methionine for 0, 18, or 36 h. PSD-95 was immunoprecipitated from the lysates, and samples were analyzed by SDS-PAGE and subsequent autoradiography. Densitometric analysis of the results is shown. Error bars, S.E. No statistical significance between KO and control was found by Student's t test (n = 3). d, cortical neurons from E15 LRP1lox/lox (Control) and NesCre/LRP1lox/lox (KO) animals were prepared. On DIV 12, neurons were pretreated with 1 μm DAPT, 10 μm L-685,458, or DMSO (1:1000; vehicle) for 2 h and were then further treated with NMDA (20 μm, 3 min). After the NMDA treatment, the cells were briefly washed with fresh medium and then cultured in fresh medium for the time indicated. Whole cell lysates were subjected to Western blotting to detect PSD-95 and actin. Actin served as a loading control. A representative blot of two independent experiments is shown. B, primary cultured cortical neurons were prepared from E15–E16 NesCreLRP1lox/lox (KO) and E15 LRP1wt/lox (Control) mouse embryos. On DIV 10–12, neurons were treated with NMDA (20 μm, 3 min). After further incubation for 10 or 20 min, whole cell lysates were prepared and subjected to immunoprecipitation (IP) with a PSD-95 antibody. Lysates, supernatants, and beads were analyzed by immunoblotting (IB) with the antibodies indicated. FK2, an antibody against mono- and polyubiquitin. Representative blots (a) and quantitative analysis (b) of five independent experiments are shown. Statistical analysis was done by Student's t test (*, p < 0.05). C, primary cultured cortical neurons were prepared from E15–E16 NesCreLRP1lox/lox (KO) and E15 LRP1wt/lox (Con) mouse embryos. On DIV 10–12, cell surface proteins were biotinylated. Subsequently, whole cell lysates were prepared, and biotinylated proteins were precipitated with Neutravidin. Aliquots from the lysates and precipitated proteins were analyzed by immunoblotting with anti-LRP1, -GluN1, -GluN2A, and -GluN2B antibodies. Actin served as a loading control. Representative blot (a) and quantitative analysis (b) of at least seven independent experiments are shown. Proteins in the lysate (total) were normalized to actin, and cell surface proteins were measured as biotinylated protein/total protein. Statistical analysis was done by Student's t test. n.s., not significant (p ≥ 0.05). D, primary cultured cortical neurons were prepared from E15–E16 NesCreLRP1lox/lox (KO) and E15 LRP1wt/lox (Control) mouse embryos. On DIV 10–12, cultures were treated with 50 μm NMDA for the time indicated or left untreated (UT). Whole cell lysates were prepared and subjected to immunoprecipitation with either α-GluN2A or α-GluN2B antibody. Input, supernatants, and precipitated proteins (beads) were analyzed by immunoblotting with the antibodies indicated. Actin served as a loading control. Representative blots (a) and quantitative analysis (b) of three independent experiments are shown. Statistical analysis was done by Student's t test (p ≥ 0.05).