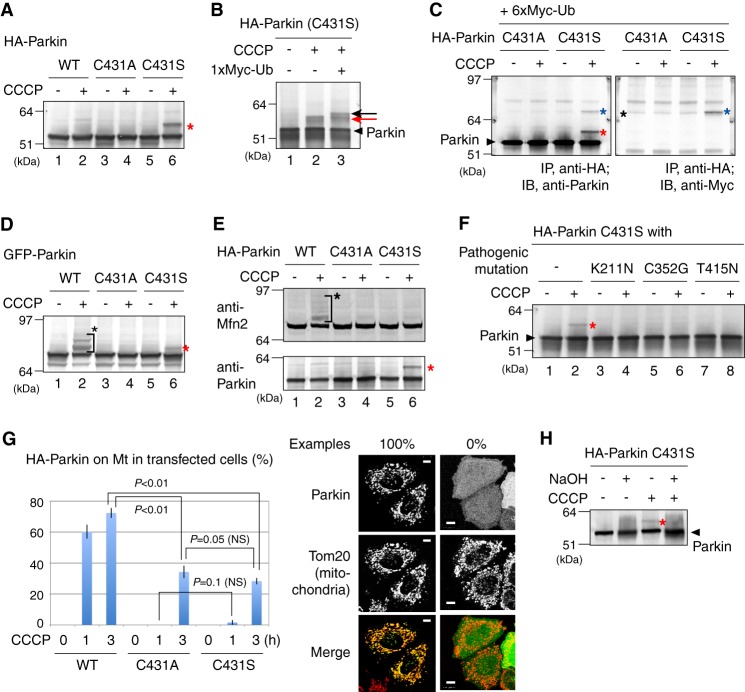
FIGURE 1.
A, a higher molecular mass population (indicated by the red asterisk) was specifically observed in the Parkin C431S mutant following CCCP treatment in HeLa cell lysates. B, immunoblotting of the Parkin C431S mutant was repeated in the absence or presence of Myc1-ubiquitin (Ub) co-expression. The slower migrating band resolved as a doublet because of the endogenous-ubiquitin adduct (indicated by a red arrow) and the Myc1-ubiquitin adduct (black arrow). C, HeLa cell lysates co-expressing HA-Parkin and Myc6-ubiquitin were immunoprecipitated with an anti-HA antibody, followed by immunoblotting with the indicated antibodies. The anti-Myc antibody specifically detected the modified Parkin(C431S) mutant. Red asterisk shows Parkin with the endogenous-ubiquitin adduct; blue asterisk shows Parkin with the exogenous Myc6-ubiquitin adduct; black asterisk indicates the cross-reacting band. D and E, Cys-431 of Parkin is important for substrate ubiquitylation. Both ubiquitylation of a pseudo-substrate (D) and a genuine substrate Mfn2 (E) were inhibited in the transfected cells by C431A and C431S mutations of Parkin. The red asterisks indicate the oxyester-linked ubiquitin and black asterisks indicate ordinary substrate ubiquitylation. F, HeLa cells expressing HA-Parkin mutants harboring the C431S mutation and one of the disease-relevant mutations (K211N, C352G, and T415N) were subjected to immunoblotting following CCCP treatment. The red asterisk indicates the ubiquitin-oxyester band. G, Parkin co-localization with mitochondria was analyzed in >100 cells per Cys-431 mutation. Example figures indicative of robust colocalization (counted as 1) and the absence of colocalization (counted as 0) are shown on the right (bars, 10 μm). Error bars represent the mean ± S.D. values of three experiments. Statistical significance was calculated using Welch's t test; NS, not significant. H, the ubiquitylated form of C431S Parkin (marked by a red asterisk) is sensitive to NaOH treatment, confirming the presence of the oxyester adduct.