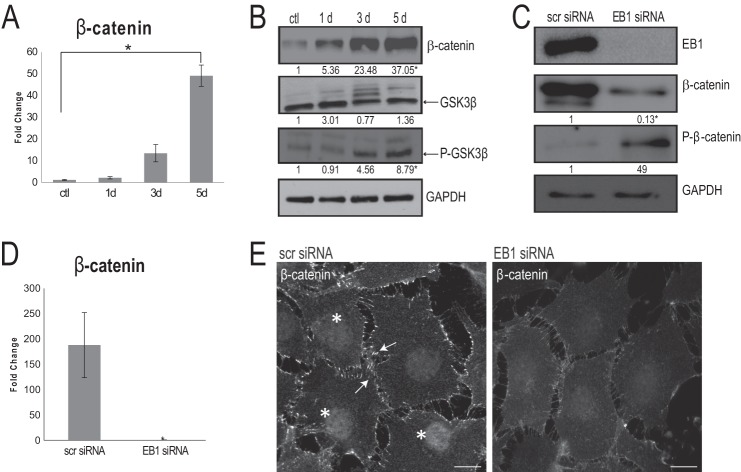
FIGURE 3.
EB1 is important for β-catenin levels and distribution during osteoblast differentiation. A, quantitative RT-PCR confirmed a significant increase in β-catenin mRNA expression levels in 5-day AA-treated osteoblasts, when compared with undifferentiated control cells (ctl). Signal intensities were normalized against GAPDH values. The time course was done in triplicate, and data are plotted as the mean ± S.D. (error bars) from three independent experiments (*, p < 0.05, Student's t test). B, time course immunoblotting analysis throughout a 5-day AA stimulation regime showed a significant increase in total β-catenin as well as a significant increase in phospho-GSK-3β (both normalized to GAPDH levels); total GSK-3β was unaltered throughout the course of AA stimulation. Numbers indicate -fold changes from control cells from three independent trials (p < 0.05, Student's t test). C, following EB1 knockdown by siRNA for 72 h, immunoblot analysis of 5-day AA-simulated osteoblast cell lysates revealed that, concomitant with EB1 reduction, there is a significant decrease in β-catenin levels and a significant increase in phospho-β-catenin levels, compared with scrambled (scr) siRNA-treated cells. Numbers indicate -fold changes from scrambled siRNA-treated cells (p < 0.05, Student's t test). D, quantitative RT-PCR, after 72-h EB1 siRNA knockdown, displayed an accompanying significant decrease in β-catenin mRNA expression levels, compared with scrambled siRNA-treated cells. Values are presented as mean ± S.D. from three independent experiments (*, p < 0.01). E, immunofluorescence analysis of 5-day AA-treated osteoblasts exposed to control, scrambled siRNA showed robust recruitment of β-catenin at the cell cortex (arrows) and within the nucleus (asterisks); however, these β-catenin localizations were visibly diminished in cells treated with EB1 siRNA. Scale bars, 10 μm.