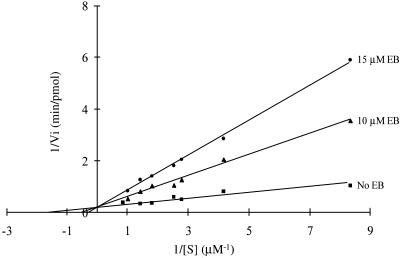
Figure 3.
Ethidium bromide exerts apparent competitive inhibitory kinetics. The initial rate of cleavage of internally 32P-labeled R1.1 RNA was determined as a function of substrate concentration at two EB concentrations. The concentration of RNase III in the assays was 12 nM (dimer concentration). The data were analyzed by plotting the reciprocal of the initial cleavage rate versus the reciprocal of the substrate concentration and generating best fit lines according to Michaelis–Menten kinetics (50). The lines shown share a common y intercept, determined by the average value of the y intercepts for each of the experiments. The Km and kcat values for R1.1 RNA cleavage in the absence of EB are 325 µM and 28 min–1. The Ki value (1.7 ± 0.3 µM) was determined as described (50).