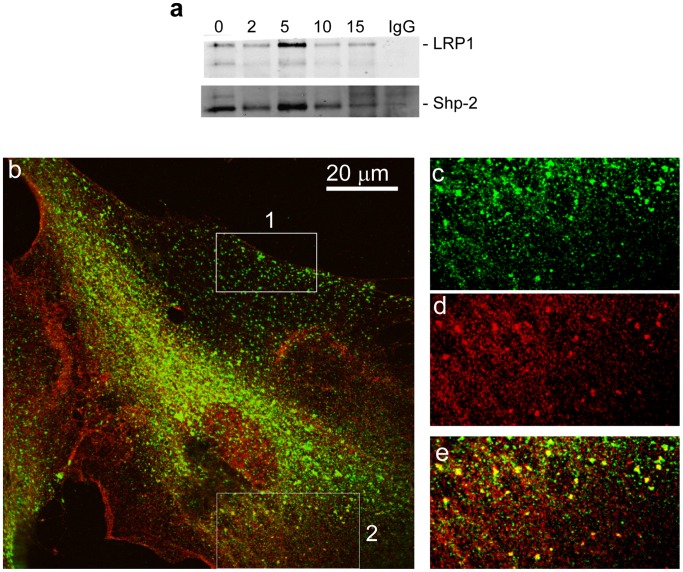
Figure 6. SHP-2 and LRP1 co-immunoprecipitate and co-localize in fibroblasts following PDGF stimulation.
(a) WI38 fibroblasts were serum starved overnight and stimulated with PDGF (30 ng/mL) for 2, 5, 10 and 15 minutes at 37°C. Cells were treated with DSP crosslinker for 30 minutes on ice prior to lysis. Lysates were immunoprecipitated with a SHP-2 antibody (sc-280) and western blot analysis was performed using a monoclonal antibody to LRP1, 11H4 (top panel). Loading was controlled by using anti-SHP-2 IgG (bottom panel). As a control, non-immune IgG (IgG) was employed for immunoprecipitation (15 min stimulation with 30 ng/ml PDGF). (b) Immunflourescence studies reveal colocalization of LRP1 and phospho-SHP-2 in fibroblasts stimulated with PDGF-BB. WI38 cells were cultured on glass cover slips, fixed with formaldehyde, and processed for immunofluorescent microscopy as described in “Methods”. The confocal image was taken using 60X oil immersion objective. Box 1 is a representative section from the ‘trailing’ edge of this migrating cell and box 2 is a representative area of the ‘leading’ edge of the cell. (c–e) represent a 3 fold enlarged area from box 2, at the ‘leading’ edge of the cell; (c) green LRP1 staining, (d) red phospho-SHP-2 staining, (e) yellow colocalization of LRP1 and phospho-SHP-2.