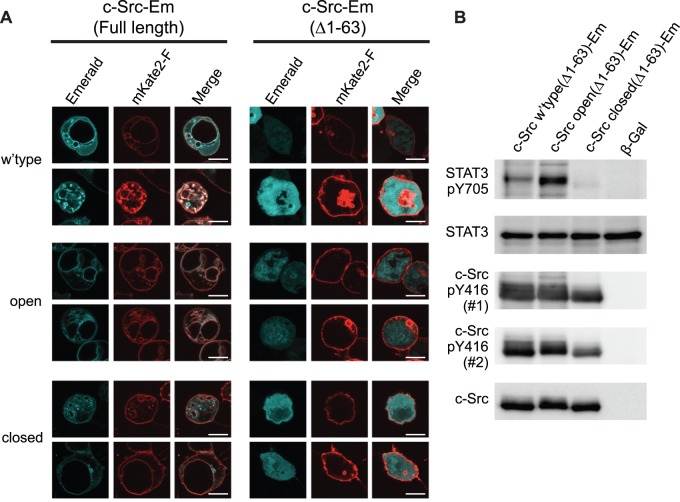
Figure 2. Phosphorylation of Y416 in the closed, repressed conformation is driven by core kinase domains.
A. Confocal micrographs of c-Src expressed as fusions to Emerald in AD293 cells in either full length or truncated form lacking the first 63 residues (Δ1–63), which contains the unique domain and myristoylation sequences. Cells were co-transfected with mKate2-F, which is a fluorescent protein containing a farnesylation signal to localize it to membranes. Turquoise shows c-Src-Em, red shows mKate2-F. Scale bar, 10 µm. Images are representative of three independent experiments. B. Western Blots of AD293 cells transfected with the indicated constructs for 24 h with 10 µg total protein lysate. Phospho-Y416 was detected with two different antibodies: #1, cat. PK1109 from Calbiochem; #2, cat. 2101 from Cell Signaling. Key: Em, Emerald fluorescent protein; closed, Q528E, P529E, G530I mutant of c-Src; open, Y527F mutant of c-Src; β-gal, β -galactosidase in same vector as c-Src constructs. Experiments were performed at least twice, which showed consistent results.