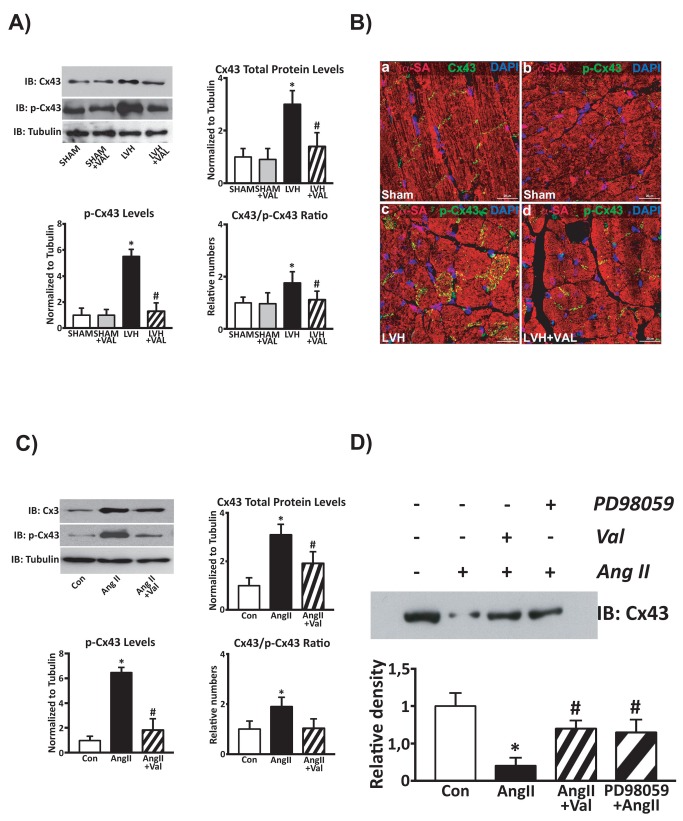
Figure 6. Phosphorylation at residues Ser279/Ser282 is associated with connexin 43 displacement.
In vivo (A) and in vitro (C) phosphorylated levels of Connexin 43. A: An increased Cx43 total protein expression along with its hyperphosphorylation at Ser279/Ser282 was observed in cardiac lysates from hypertrophic hearts compared to SHAM and SHAM+VAL, while chronic administration of Valsartan reduced both these two molecular responses; *p<0.05 vs. Sham; #p<0.05 vs. LVH. B: high-magnification immunohistochemistry and confocal representative images (red: α-sarcomeric actin, α-SA; green: Cx43 or p-Cx43; blue: DAPI) show normal Cx43 localization within gap-junction (panel a) and the very little, if not negligible, levels of Ser279/Ser282-phosphorylated Cx43 (panel b) in rat SHAM hearts. LVH by pressure overload is associated with an intense Cx43 phosphorylation at Ser279/282 and its displacement from gap junction (panel c). The latter is significantly attenuated by VAL treatment (panel d). C: Increased protein levels and hyper-phosphorylation of Connexin 43 in cardiomyocytes treated with Angiotensin II (AngII, 5µmol/L) compared to un-stimulated cardiomyocytes, with a significant reduction after concurrent valsartan administration (10µmol/L, *p<0.05 vs. Con; #p<0.05 vs. AngII). D: Representative western blot of Cx43 total protein levels in lysates from membrane fractions of cultured cardiomyocytes. After AngII challenge, Cx43 was displaced from the gap junction as demonstrated by its reduced levels in the membrane fractions (lane 2) compared to un-stimulated cells, whereas Valsartan (lane 3) and the MAPK/ERK1/2 kinase inhibitor PD98059 (lane 4) both reduced Cx43 gap junction displacement in AngII-treated myocytes.