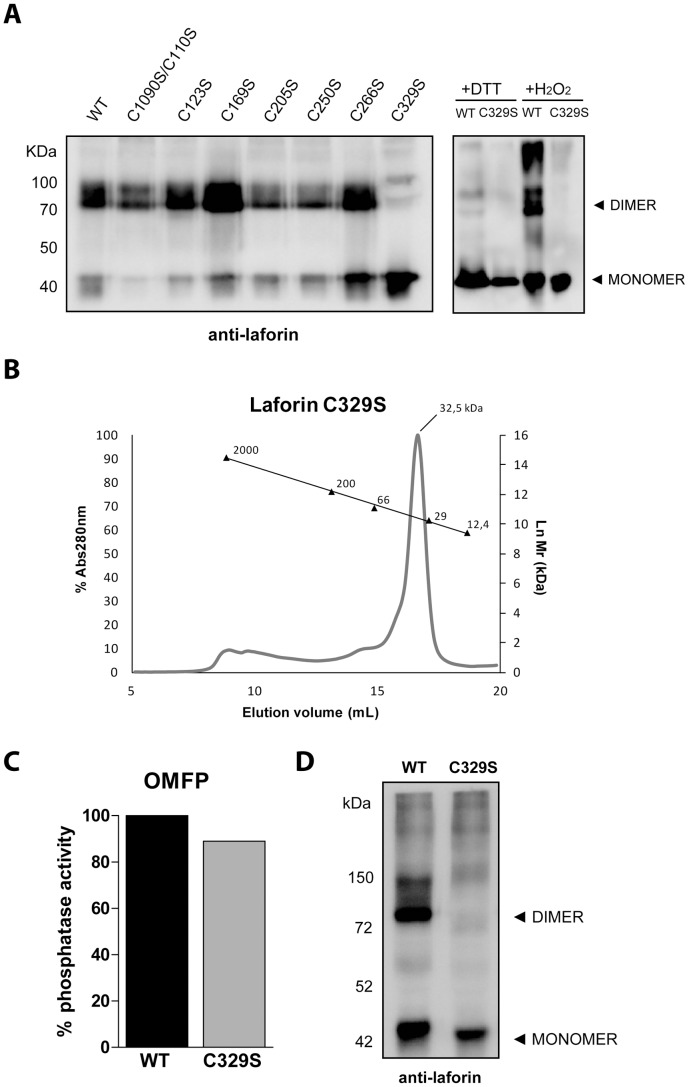
Figure 3. Laforin-C329S is monomeric.
(A) In vitro dimerization assay. Recombinant proteins expressed in bacteria were purified and subjected to non-reducing, non-denaturing electrophoresis and were immunodetected using anti-laforin antibody. All mutants could form dimers with the exception of C329S (left panel). WT laforin and C329S were further analyzed by incubation in the presence of 10 mM DTT or 10 mM H2O2 and analyzed for the presence of monomeric and dimeric species (right panel). (B) Laforin C329S exclusion chromatography analysis. A single peak is observed in an elution volume corresponding to 32.5 kDa (molecular size markers are indicated). (C) In vitro phosphatase activity assay of the same sample showed that the C329S mutant displayed a catalytic activity comparable to the wild type protein. (D) In vitro dimerization assay of laforin-C329S expressed in mammalian cells. Crude lysates from HEK293 cells transfected with myc-laforin (wt, wild type) or myc-C329S plasmids were analyzed by Western blot. The electrophoresis was carried out in non-reducing, non-denaturing conditions, and the immunodetection was performed using anti-laforin antibody. The position of the monomeric and dimeric forms of laforin is indicated.