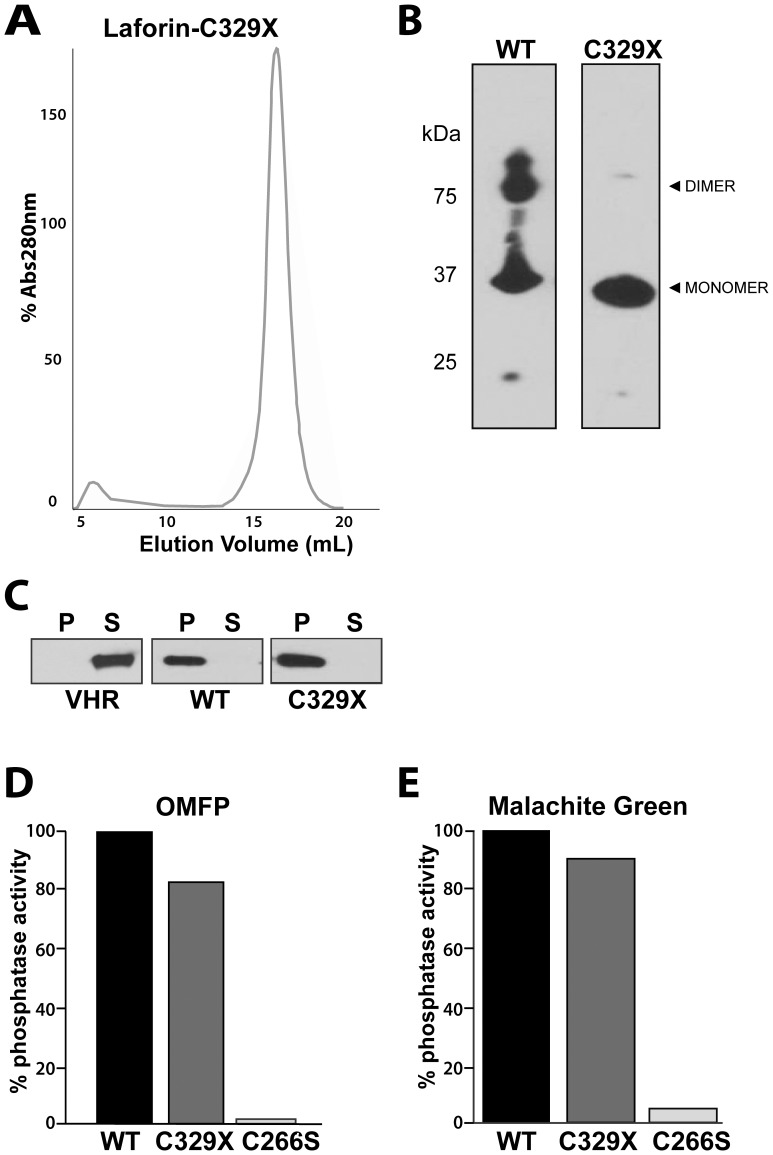
Figure 4. Laforin-C329X does not dimerize, but maintains activity.
(A) Laforin-C329X exclusion chromatography chromatogram. Recombinant laforin-C329X was purified via affinity chromatography and then subjected a HiLoad 16/60 Superdex 200 sizing column. A single peak elutes that corresponds to 32.5 kDA. (B) In vitro dimerization assay. Recombinant laforin-WT and laforin-C329X were purified via affinity chromatography, subjected to non-reducing, non-denaturing electrophoresis, and immunoblotted using anti-HIS antibody. (C) Co-sedimentation assay of protein and amylopectin (amylopectin binding assay). Recombinant histidine-tagged proteins were incubated with 5 mg/ml amylopectin, amylopectin was pelleted by ultracentrifugation, proteins in the pellet (P) and supernatant (S) were separated by SDS-PAGE, and visualized by Western analysis. Amylopectin-bound proteins are found in the pellet (P) and unbound proteins are found in the supernatant (S).VHR, Vaccinia virus phosphatase VH1-related; WT, laforin; C329X, laforin-C329X. (D) Recombinant laforin-WT, laforin-C329X, and laforin-C266S were purified and used for in vitro phosphatase assays employing the artificial substrate OMFP. The mutant C266S was used as a negative control. (E) Recombinant laforin-WT, laforin-C329X, and laforin-C266S were purified and used for in vitro phosphatase assays employing the phosphorylated glucan amylopectin. Inorganic phosphate release was detected via malachite green.