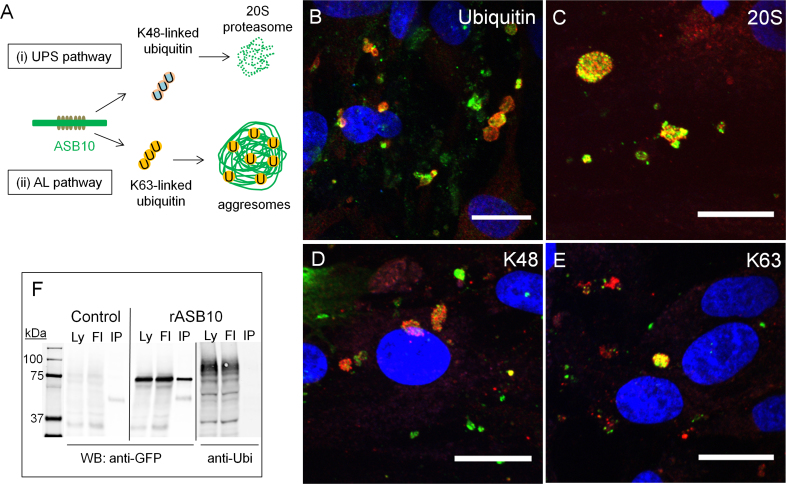
Figure 3.
Ankyrin repeat and suppressor of cytokine signaling box containing protein-10 colocalizes with ubiquitin-mediated degradation pathway biomarkers in human trabecular meshwork cells. A: A schematic of ubiquitin-mediated degradation pathways are shown. B–E: Human trabecular meshwork (HTM) cells were immunostained with the rabbit polyclonal ankyrin repeat and suppressor of cytokine signaling (SOCS) box containing protein-10 (ASB10) antibody (red; B, C) and ubiquitin (green; B), the alpha 4 subunit of the 20S proteasome (green; C), the goat polyclonal ASB10 antibody (red; D, E), K48-linked ubiquitin (green; D), or K63-linked (K63; E) ubiquitin rabbit monoclonal antibodies. Nuclei were stained with 4',6-diamidino-2-phenylindole dihydrochloride (DAPI). Scale bars=20 µm. F: Coimmunoprecipitation of recombinant ASB10 variant 3 (rASB10) transfected into HTM cells are shown. rASB10 was immunoprecipitated using the green fluorescent protein (GFP) antibody. Western immunoblots show that ubiquitin (Ubi) is present in the cell lysates (Ly) and in the flow through (Fl), but not in the bound lane (IP), while rASB10 is present in all lanes, as detected with the GFP antibody. Untransfected HTM cells (control) show low levels of non-specific background immunostaining by the GFP antibody. Molecular weight markers in kDa are shown.