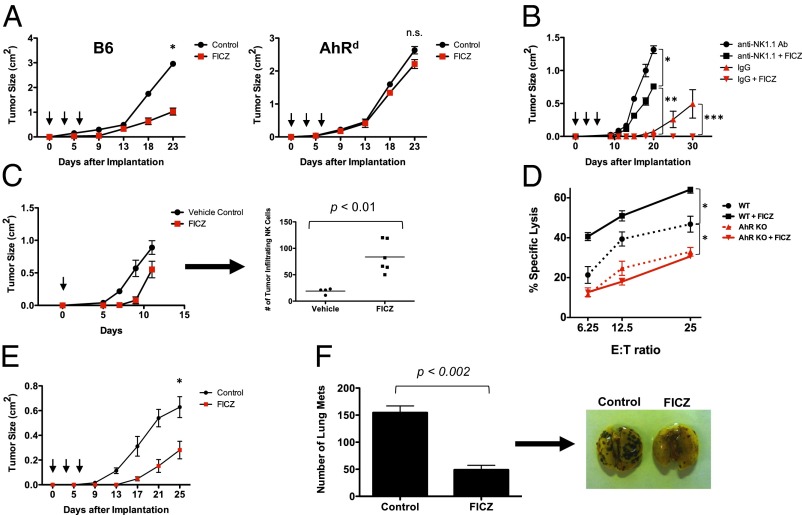
Fig. 3.
Activation of AhR enhances NK cell-mediated control of tumor growth. (A) RMA-S cells (3 × 105) were implanted on WT and AhRd mice (n = 6 per cohort), then treated with FICZ (3 μg per mouse, i.p.) or vehicle control on days 0, 2, and 4 (arrows) (*P < 0.001; n.s., not significant). These results were reproduced at least once. (B) Rag1−/− mice (n = 4 per cohort) were implanted with RMA-S cells (1 × 105) and treated with anti-NK1.1 antibody (PK136) i.p. to deplete NK cells or IgG control antibody. The mice were then treated with FICZ (3 μg per mouse, i.p.) or vehicle control on days 0, 2, and 4. Tumor growth is plotted (*P < 0.002; **P < 0.0001; ***P < 0.05). (C) RMA-S tumor cells (1 × 106) were implanted into the s.c. compartment in Rag1−/− mice. FICZ was administered i.p. once at day 0. Tumors were harvested at day 12, dissociated, and analyzed by FACS for tumor-infiltrating NKp46+ cells. These results were reproduced at least once. (D) Magnetic bead-enriched NK cells from AhR−/− and AhR+/+ mice were cultured in IL-2 (1,000 U/mL) for 7 d, either with FICZ or vehicle control. The NK cells were then incubated for 4 h with RMA-S target cells in a calcein-release cytotoxicity assay (*P < 0.05). There were no statistically significant differences in cytotoxicity between the AhR KO and AhR KO + FICZ groups. Results were reproduced at least once. (E) Rag1−/− mice (n = 8 per cohort) were implanted with B16 melanoma cells (1 × 105) and treated with FICZ or vehicle control, as above (*P < 0.05). These results were reproduced at least once. (F) Rag1−/− mice (n = 3 per cohort) were injected with B16 cells (5 × 105) via tail vein and then treated with FICZ or vehicle control, as above. Lungs were harvested and fixed in Bouin’s solution, and metastatic implants were counted. Representative images of lungs are shown.