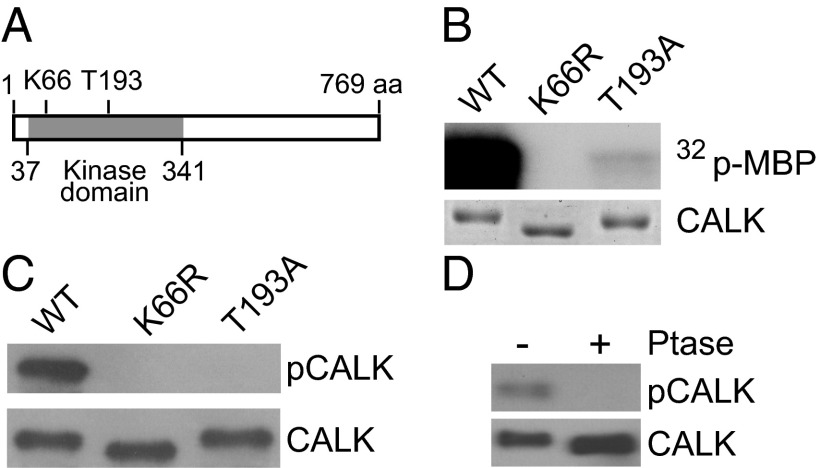
Fig. 1.
T-loop T193 phosphorylation is required for CALK activity. (A) A diagram of CALK domain structures showing the protein kinase domain and residues critical for CALK activity. (B) T193 is required for CALK activity. Bacterially expressed WT and mutated forms of CALK were assayed for kinase activity in vitro with MBP as substrate. SDS/PAGE analysis with Coomassie blue staining showed equal loading. (C) T193 is phosphorylated in WT CALK but not in kinase-dead and T-loop mutants. Bacterially expressed WT and mutated forms of CALK were analyzed by immunoblotting with anti-pCALK and CALK antibodies. pCALK stains CALK phosphorylated at T193, whereas CALK stains total CALK. (D) Endogenous CALK T193 is phosphorylated at steady state. Steady-state cell lysates were incubated with phosphatase followed by immunoblot analysis with anti-pCALK and CALK antibodies.